In this post
A compound is made up of two or more atoms of different elements chemically bonded together. This chemical bonding may occur through the transfer of electrons to form ions which bond through ionic bonds, as is the case with ionic compounds such as sodium chloride. It may also occur through the sharing of electrons in covalent bonds, found in simple molecular covalent compounds such as oxygen gas and giant molecular covalent compounds such as diamond. Generally, organic compounds contain at least one carbon atom. However, most of the organic compounds we study contain both carbon and hydrogen atoms, along with other elements such as oxygen or nitrogen.
Carbon atoms have four electrons in their outer shell. In order to achieve stability, they share these electrons with other atoms such as other carbon atoms, hydrogen, oxygen and nitrogen.
This means that carbon can form a large range of organic compounds with different shapes, structures and chain lengths. Carbon compounds include ring structures, long and short straight chains and branched chains. An organic compound which contains only hydrogen and carbon atoms is known as a hydrocarbon.
Key terms
Organic compound – a compound that contains at least one carbon atom.
Hydrocarbon – a compound that consists of only carbon and hydrogen.
Representing organic compounds
A chemical formula gives information about the identities of elements present in a molecule. There are many types of formulae used to represent organic molecules including empirical formulae, molecular formulae, general formulae, structural formulae and displayed formulae. You need to understand how to use each of these formulae to represent the organic molecules covered in this unit.
Molecular formulae
The molecular formula of a compound shows the actual number of atoms of each element present in a molecule of that compound. For example, the molecular formula of propane is . This means that each molecule of propane contains three carbon atoms and eight hydrogen atoms.
Empirical formulae
The empirical formula of a compound shows the simplest whole number ratio of atoms of each element present in a compound. The molecular formula of a compound is always either the same or a simple multiple of the empirical formula.
For example, the molecular formula of propane is C3H8. The ratio of 3:8 cannot be simplified to any other whole number ratio, therefore the empirical formula of propane is also C3H8. The molecular formula of ethane is C2H6. This ratio of 2:6 can be simplified to 1:3. Therefore, the empirical formula of ethane is CH3.
General formulae
The general formula is a type of empirical formula used to represent the common composition of a molecule of any given class of compounds. The general formula allows us to predict the molecular formula for any compound from a given class.
Functional group
A functional group is a group of atoms bonded together in a compound which are responsible for the chemical behaviour of that compound.
Homologous series
A homologous series is a family of hydrocarbon compounds which share the same functional group, same general formula and similar chemical properties. Each successive member of a homologous series differs from the previous by having an extra CH2 group i.e. they possess one more carbon atom and two more hydrogen atoms than the previous member of the series. Examples of homologous series introduced in this topic include:
Molecular formulae, empirical formulae and general formulae only provide information about the atoms of each element present in a compound. They do not show how these atoms are bonded to each other and therefore do not provide information about the structure of the molecule. Molecular structures can be shown by the structural and displayed formulae.
Structural formula
The structural formula of a compound is a written description showing the order in which the atoms and groups of atoms are arranged in a molecule. For example, the structural formula of propane is CH3CH2CH3.
Displayed formulae
The displayed formula of a compound is a 2-D diagram showing how the atoms and groups of atoms are arranged as well as the covalent bonds present in a molecule. In a displayed formula, each element is represented using its chemical symbol and each covalent bond is represented as a single line. Each single line represents a single pair of shared electrons. Every bond between all atoms must be drawn in a displayed formula. The displayed diagram does not show the 3-D structure of the molecule, it simply shows the molecule as a straight and flat structure.
Naming organic compounds
Chemical compounds are named using the rules of International Union of Pure and Applied Chemistry (IUPAC) nomenclature. This is an international set of rules used to make sure that chemists all over the world name compounds using the same method.
You should be able to use these rules to name the organic compounds covered in this specification which contain up to six carbon atoms. It is important to remember that when naming organic compounds:
- Numbers are separated from each other using commas
- Numbers and letters are separated using dashes
The general method for deducing the IUPAC name of any organic compound is:
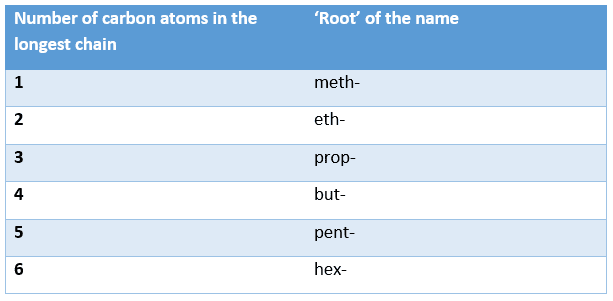
- Count the number of carbon atoms in the longest consecutive chain.
The ‘root’ of the name is the first part of the compound’s name and is based on the number of carbon atoms in the longest chain. The ‘roots’ used for compounds containing up to six carbon atoms are shown in the table below:
- Identify the functional group present to deduce the ‘suffix’.
The ‘suffix’ is the ending of the compound’s name. The suffix changes depending on the functional group present. The suffixes for the homologous series covered in this specification are shown in the following table:
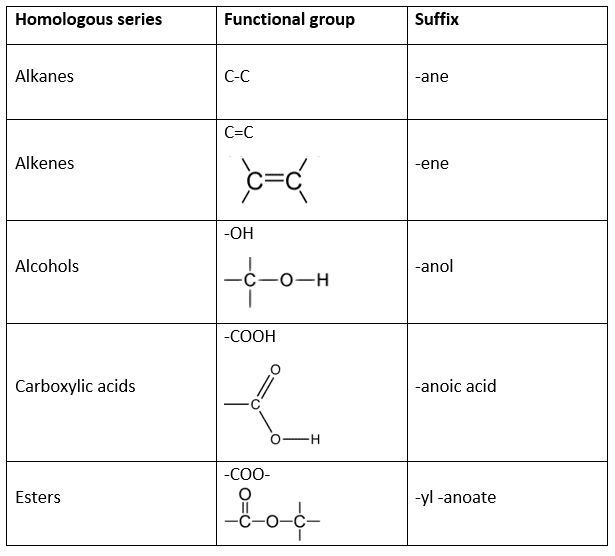
Alkanes – the alkane family of hydrocarbons have only single bonds between all the carbon atoms. Any compound of this type is coded with a suffAix of ‘-ane’, for example, ethane is a hydrocarbon with all the carbon atoms having single bonds. We can also tell that ethane has two carbon atoms because of the ‘-eth’ base. Ethane has the structural formula .
Alkenes – the second family of hydrocarbons uses the suffix ‘-ene’ and are known as alkenes. These have at least one set of double bonded carbon atoms. For example, the compound ethene has two carbon atoms but, due to the suffix, we know this must have a double carbon bond.
- Identify the position of any substituents present.
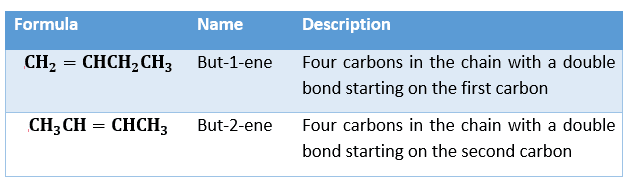
Substituents are groups of atoms which are attached to the central carbon chain. These may be functional groups or side chains. The carbon atoms in the longest chain should be numbered so that any substituent is attached to the carbon atom with the lowest possible number. The number of the carbon atom to which the substituent is attached is known as the ‘locant’ and this may be given at the start or in the middle of the compound’s name depending on the type of functional group present. For example, the position of a double bond in an alkene. Inserting a number between the root and suffix, we can identify the carbon atom that the double starts from.
Side chains
The carbon chains in hydrocarbon compounds can be straight chains only but they may also have smaller side branches attached. When naming branched hydrocarbon compounds, the size and position of any side branches must be given. Chains can be very long and have smaller branches on them.
The suffix of the name is based on the number of carbon atoms in the longest consecutive chain. The prefix of the name is based on the position and size of the side branches. The position of the side chain is then shown using the same numbering system as we used before, where the number represents the carbon atom which the side chain is attached to. The carbon atoms are always numbered so that the side branches or functional groups are attached to a carbon atom with as low a number as possible.
Two side chains found in common hydrocarbons are the methyl and ethyl groups. The methyl side chain contains one carbon atom attached to three hydrogen atoms (CH3) and the ethyl side chain contains two carbon atoms with five hydrogen atoms attached (CH3CH2).
For example, the hydrocarbon compound shown in the diagram below contains four carbon atoms in the longest consecutive chain. The suffix for this compound’s name would therefore be butane.
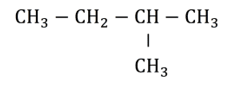
There is a methyl group attached to the second carbon in the chain. Remember the carbon atoms are numbered so that the side chains are attached to the lowest possible numbered carbon atom. This may involve counting the carbons from left to right or, as in this case, right to left. The prefix of the name is therefore 2-methyl. The IUPAC name for this hydrocarbon compound is therefore 2-methylbutane.
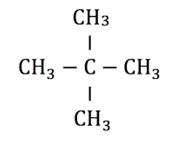
In the molecule pictured above the longest chain contains three carbon atoms with only single covalent bonds between them. The suffix would therefore be propane. If the side branches are identical, the number of them present can be indicated using the prefixes di- for two or tri- for three. There are two methyl branches attached to the second carbon atom in the chain, so the prefix of the name would be 2,2-dimethyl. The overall name of this compound is therefore 2,2-dimethypropane.