In this post
Everything around us is made up of particles. The arrangement of particles differs in the three states of matter – solids, liquids and gases. These different arrangements of particles can be used to explain the differences in their properties and behaviours.
Solids
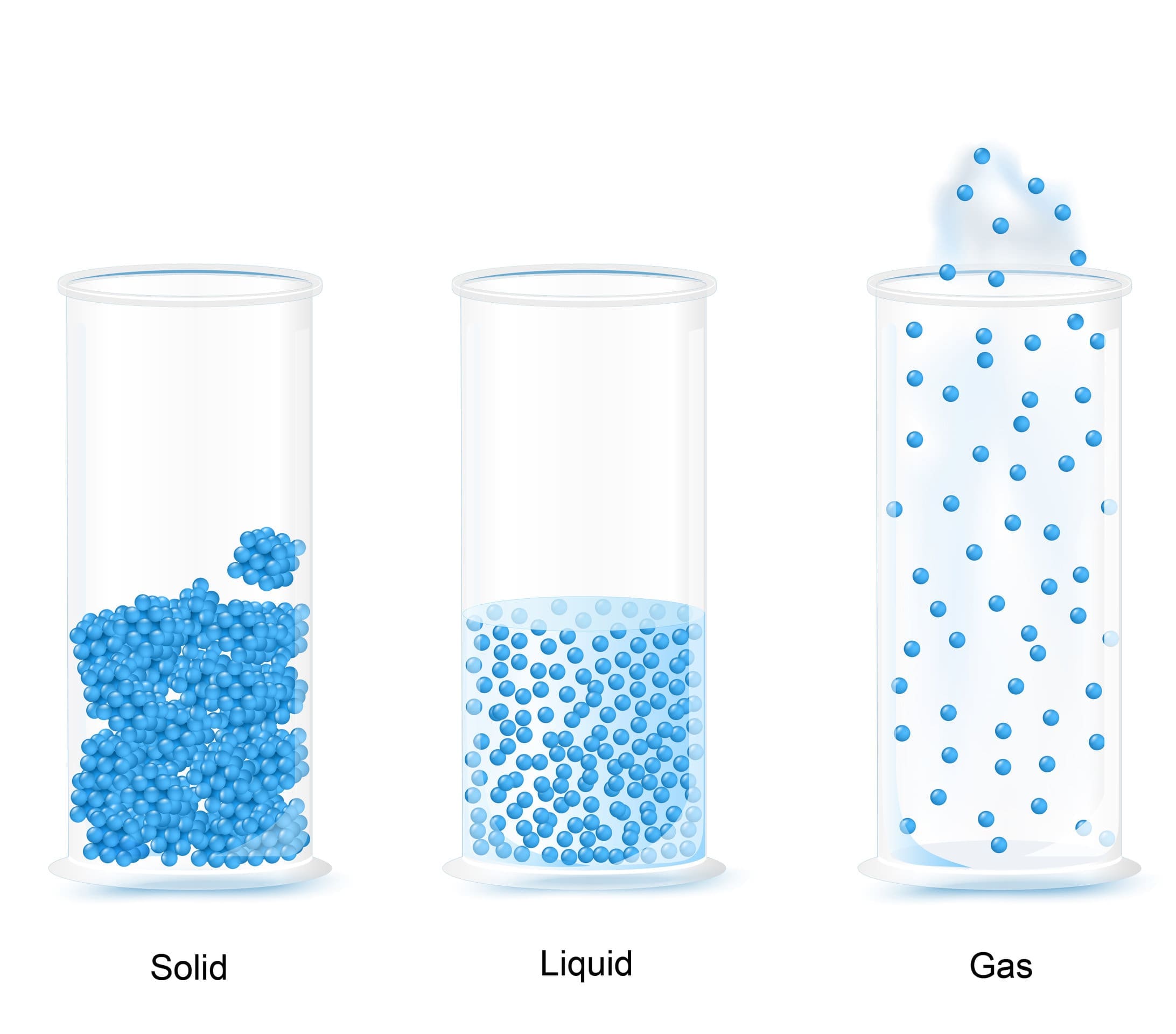
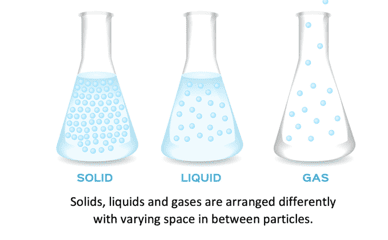
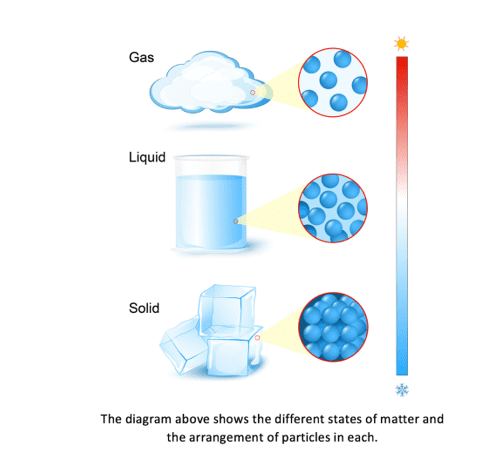
The particles in solids have strong forces of attraction between them. This means that solids have fixed shapes as their particles are very close together with a regular arrangement. As the particles are fixed tightly together, with no spaces between them, they cannot move or flow, and solids cannot be compressed. The particles in a solid can only vibrate in their fixed position.
Liquids
The particles in a liquid are still close together but the forces between the particles are weaker than those between the particles in a solid. In a liquid, the particles move continually in a random arrangement, meaning there is no specific pattern of their movement. This movement creates gaps between the particles which other particles are able to move into. As the particles in liquids can therefore move past each other, liquids do not have a fixed shape and are able to flow.
Gases
The particles in a gas have very weak forces of attraction between them. This allows the particles to move constantly in a random pattern. There are very big gaps between the particles in a gas which means that the particles can move easily past each other and gases can flow. Gases do not have a fixed shape or volume and this enables them to flow and fully fill a container as they travel quickly in different directions. When a gas is heated, the particles in a gas gain kinetic energy and move faster. This makes the gaps between the particles larger, causing the gas to expand and the pressure of the gas to increase.
Changes of state
The arrangement of particles in solids, liquids and gases is shown in the diagram below:
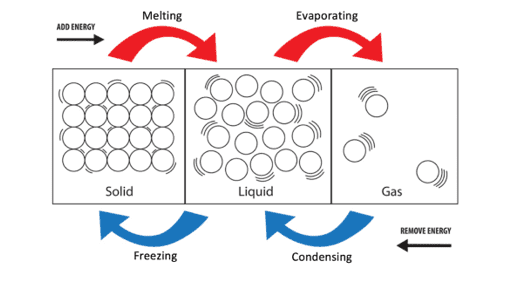
Substances can change state when they are heated or cooled. The change of state may occur between solids and liquids, liquids and gases or solids and gases as shown in the diagram below:
Solids to liquids
When a solid is heated thermal energy is transferred to particles as kinetic energy and they vibrate in their fixed positions. The more heat energy is provided, the more kinetic energy is provided and the more the particles vibrate. Once enough heat energy is provided, the vibrations of the particles may be so vigorous that the forces of attraction between them are too weak to hold them in place any longer. The particles move free from their fixed positions and gaps form between them. This causes the solid to expand and turn into a liquid. For example, if an ice cube is heated, the thermal energy is transferred to kinetic energy and the particles start to move more. The ice cube melts to liquid water if enough heat energy is provided. The temperature at which a solid becomes a liquid is known as its melting point.
Liquids to solids
When a liquid is cooled the thermal energy is transferred from the liquid to the surroundings. The particles have less kinetic energy and begin to move slower. The distance between the particles decreases and the particles become so close that the forces of attraction between them increase in strength. The particles are held closer together. If enough heat energy is removed, the liquid turns into a solid. The temperature at which the liquid becomes a solid is known as the freezing point.
Liquid to gas
When thermal energy is provided to a liquid, the particles gain kinetic energy and move faster. The faster the particles move, the bigger the gaps between the particles and the weaker the forces of attraction between them. The liquid expands. If enough thermal energy is provided, all of the forces of attraction between the liquid particles are overcome. The particles are moving at such a speed that they are able to leave the liquid. This process by which the particles leave the liquid is known as evaporation.
If thermal energy continues to be provided, the particles move faster and faster and a change of state occurs where the liquid becomes a gas. The temperature at which the liquid becomes a gas is known as the boiling point. The process of evaporation occurs at a temperature just below the boiling point of the liquid.
Gas to liquid
If the gas was to be cooled again, by removing the thermal energy, the kinetic energy of the particles is decreased. The gas particles begin moving slow enough so that forces of attraction form between them. If the gas is cooled enough, the forces between the particles will be strong enough to hold them closer together and the gas becomes a liquid. This process is known as condensing.
Solid to gas
Under specific conditions, some solids are able to turn directly into a gas without becoming a liquid first. For example, solid carbon dioxide (dry ice) is converted into gaseous carbon dioxide at a temperature of -78. This process is known as sublimation.
The arrangement of particles and changes of state which occur are shown in the diagram below:
If the substance is pure, it has a sharp and specific melting and boiling point. If the substance is a mixture, the melting and boiling points will occur over a range of values. For a mixture, the melting and boiling points are determined by the ratio of different atoms contained.
The temperature at which the melting point and boiling occur is measured in the unit degrees Celsius (oC). Every substance has a different melting and boiling point. The melting point and freezing point occur at equal temperatures. The temperature at which boiling and condensing occur is also the same.
For example, the temperature at which ice melts and water freezes is 0℃. The temperature at which the water boils and steam condenses is 100℃.
The table below summarises the changes of state, changes in their particle arrangement and motion
| Change of state | Description of change of state | Changes in the arrangement of particles | Changes in the motion of the particles |
| Melting | Solid to liquid | Regular and fixed to random | Vibration at a fixed point to random movement |
| Evaporating/boiling | Liquid to gas | Becomes more random | Fast random movement |
| Condensing | Gas to liquid | Particles get closer but remain random | Random movement which slows down |
| Freezing | Liquid to solid | Random to fixed and regular | Movement becomes vibrations around a fixed point |
Temperature-time graphs
When a change of state occurs, there is no change in the size or shapes of the particles. The particles simply move so only their arrangement or motion alters. The lack of change in the particles means that there is no change in the mass of the substance. This occurs according to the principle of conservation of mass. For example, 10 g of water boils to form 10 g of steam, or freezes to form 10 g of ice. No mass is lost or gained.
When a substance absorbs heat energy it can melt or boil depending upon its current state. The temperature of the substance does not change during the change of state even though energy is still being transferred. You can conduct an experiment to prove this. This temperature of the substance can be monitored over time. A temperature-time graph can be produced which shows temperature plotted against the amount of energy absorbed as shown in the diagram below:
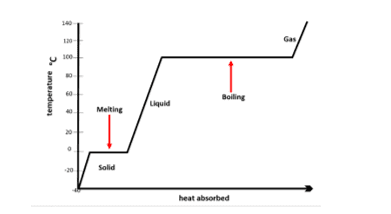
As shown on the graph, the temperature stays the same during melting even though heat energy is still being absorbed. The temperature also stays the same while liquid freezes, even though heat energy is still being released to its surroundings.