In this post
The amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. These experiments often involve measuring the temperature changes that occur in water or aqueous solutions during these reactions. The calorimetry experiments for these four types of reactions will now be described.
Combustion
Combustion reactions are exothermic reactions. A fuel is a substance which releases heat energy when it is burned. Different fuels will release different amounts of heat energy. The heat energy released by the fuel is transferred to the surroundings.
To measure the heat energy released by a fuel, we burn a sample of the fuel with a known mass, below a glass or metal container full of water. The glass or metal container is known as a calorimeter.
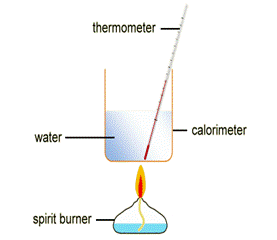
The heat energy released from the combustion of the fuel is transferred to the water, causing the temperature of the water to increase. The method used to determine this temperature increase is described below:
- 50cm3 of cold water is measured into a calorimeter.
- The initial temperature of the water is measured and recorded.
- A spirit burner containing the fuel is placed below the calorimeter and the wick is lit using a lit splint.
- The fuel is burned for a set amount of time (usually a couple of minutes).
- The highest temperature reached by the water is recorded.
During combustion reactions, the heat energy produced is transferred to the surroundings. This means that not all of the heat energy will be transferred directly into the water. Some will also be transferred to the container itself, as well as the air surrounding the container. The following steps should be taken to minimise this heat loss, and achieve measurements which are as accurate as possible:
- Use of shielding around the spirit burner to reduce heat loss to the air
- Use of a lid on the calorimeter to reduce heat loss through convection
- Use as small a distance as possible between the flame and container to reduce convection
When comparing the heat energy transferred by different fuels it is important that everything is kept the same in order to make it a fair test. The distance between the flame and calorimeter, the type of calorimeter used, the volume of water used and the amount of time the fuel is burned for should all be kept the same for each experiment completed.
Displacement reactions
A displacement reaction is one in which a more reactive metal replaces a less reactive metal in a compound. Displacement reactions are exothermic, so heat energy is released to the surroundings. If the metal added is in solid form and the metal compound is present as an aqueous solution, the amount of heat energy released can be calculated using the measured temperature increase of the solution of the metal compound.
The method used to determine the temperature increase is described below using the addition of zinc powder to copper sulphate solution as an example:
- Measure 50cm3 of the copper sulphate solution into a polystyrene cup or glass beaker.
- Measure and record the initial temperature of the copper sulphate solution.
- Add a known mass of zinc powder to the copper sulphate solution and stir.
- Measure the temperature continually and record the highest temperature reached by the solution.
Dissolving
Dissolving occurs when a solid is added to a liquid and the bonds between the particles in the solid are broken, causing the solid particles to separate and spread out between the liquid particles.
One common type of dissolving experiment used is salts dissolving in water. Salts are ionic compounds. When they dissolve in water, the ions separate. Energy is required to overcome the forces of attraction between the ions in the solid form. This part of dissolving is endothermic.
When the ions have separated, they are able to spread out between the water particles. Forces of attraction are formed between the ions and the water molecules. This forming of the forces of attraction releases heat energy and is exothermic.
The dissolving of salts can be either an endothermic or exothermic change depending on how much energy is taken in to separate the ions in the solid compared to the energy released when the forces of attraction are formed between the ions and water molecules. The method for the calorimetry experiment is as follows:
- Place a polystyrene cup in a beaker.
- Measure and transfer 50cm3 of water into the cup and measure the initial temperature.
- Add a known mass of the solid solute to the water in the cup and stir the solution to dissolve the solid.
- Once the solid has dissolved, measure and record the final temperature reached by the water.
Neutralisation
A neutralisation reaction is one in which an acid reacts with an alkali to produce water and a salt. For example, hydrochloric acid and sodium hydroxide solution react together, forming water and sodium chloride solution.
Neutralisation reactions are exothermic as the system releases heat energy to the surroundings. The system in a neutralisation reaction is the acid and alkali. The surroundings are the solution in which they are contained. When the acid and alkali react, they release heat energy into the surroundings, meaning that the temperature of the solution increases.
The temperature increase of the solution can be measured and used to calculate the amount of heat energy transferred during the reaction. The method for this experiment is describe below:
- Measure out 25cm3 of the acid solution with a known concentration into a polystyrene cup. Place the cup into a glass beaker to prevent the cup being knocked over during the experiment.
- Measure and record the initial temperature of the acid.
- Measure out 25cm3 of the alkali solution. This alkali solution should have the same concentration as the acid solution to make sure that the number of moles of acid and alkali used are equal.
- Add the 25cm3 of alkali solution into the polystyrene cup and stir the solution.
- Measure and record the highest temperature reached by the solution.
Calculating the heat energy change
Once the temperature change has been measured from the calorimetry experiment, the amount of heat energy transferred can be calculated using the expression:
Energy transferred (Q) = mass of water (m) x specific heat capacity (c) x temperature change (△T)
or:
Q = mc△T
where:
= energy transferred (in J)
= mass of water or aqueous solution (in g)
= specific heat capacity of water (4.2J/goC)
= temperature change (in oC)
The mass of water or aqueous solution used can be calculated using its volume and the density. The density of water is 1g/cm3. This means that 1cm3 of water has a mass of 1g. 50cm3 would therefore have a mass of 50g. Aqueous solutions are those made using water as a solvent. We therefore use the density of water to measure the mass of these solutions. For example, 50cm3 of an aqueous solution would have a mass of 50g.
The specific heat capacity of a substance is the amount of energy needed to raise the temperature of 1g of the substance by 1oC. The specific heat capacity of water is taken to be 4.2J/g. This means that it takes 4.2 Joules (J) of energy to raise the temperature of 1g of water by 1oC.
The temperature change is the increase or decrease in temperature of the water or solution. This is calculated by subtracting the initial temperature from the final temperature. The heat energy change equation essentially tells us that the amount of heat energy transferred is equal to the mass of the water or solution that is heated, multiplied by the specific heat capacity of water (this is a constant and is equal to 4.2 joules for 1 gram of water) multiplied by the temperature change.
The heat energy change for any calorimetry experiment can be calculated using this equation. Worked example 1 on the next page shows how the equation can be applied and used in the example of a combustion reaction.
Example 1
Ethanol in a spirit burner is burned below a calorimeter containing 50cm3 of water. The initial temperature of the water was 21.5oC and the highest temperature reached after heating was 82.5oC. Calculate the heat energy change for the combustion of ethanol.
- Calculate the temperature change for the reaction.
The temperature of the water in the calorimeter before heating is 21.5. The temperature of this after heating is 82.5 To work out the temperature change we subtract the temperature before heating (21.5) from the temperature after heating (52.5).
82.5 – 21.5 = 61.0
- Substitute the values into the heat energy change equation:
Q = mc△T
Q = 50 x 4.2 x 31 = 12810J



