In this post
Testing for positively charged ions
We can conduct experiments on positively charged ions (cations) to help us see which elements are present and to also examine the properties of these. To identify the ammonium ion, NH4+, add aqueous sodium hydroxide to the solid or solution, test and heat up the mixture. If ammonium ions are present, then a strong-smelling gas will be produced. The gas that’s been produced will turn damp red litmus paper blue. This is ammonia, NH3. Below is the equation for this:
![Rendered by QuickLaTeX.com \[NH_{4^+}(aq) + OH^- \rightarrow NH_3 + H_2O\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-006b13bf7badd3671f93d50ea1b09611_l3.png?lossy=2&strip=1&webp=1)
Identifying metal cations by using precipitation reactions
As most metal hydroxides are insoluble, they can be precipitated from aqueous solutions of metal salts by adding an aqueous solution of sodium hydroxide. A precipitate is an insoluble solid that has been formed in the reaction. Precipitates come in the form of small particles that have been suspended in a solution. This may have been formed when a few drops of sodium hydroxide are added to a solution of a metal compound.
Examples
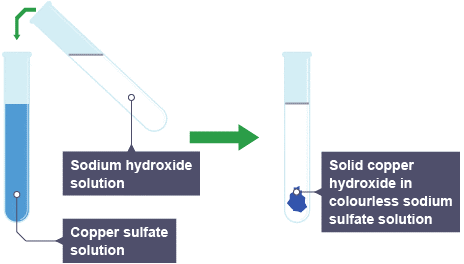
A blue precipitate of copper(II) hydroxide is formed when sodium hydroxide solution is added to copper(II) sulphate solution. Below are the equations for this:
![Rendered by QuickLaTeX.com \[CuSO_4 (aq) + 2NaOH (aq) \rightarrow Cu(OH)_2(s) + Na_2SO_4 (aq)\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-604dab503aee237dde636c2396202b28_l3.png?lossy=2&strip=1&webp=1)
Calcium, magnesium and aluminium
When calcium, magnesium and aluminium react with sodium hydroxide, they form white precipitates. You can find out whether the white precipitate is due to the presence of aluminium ions by adding more sodium hydroxide solution than required. The excess sodium hydroxide causes a precipitate of aluminium hydroxide to dissolve. The precipitates formed by calcium and magnesium ions do not dissolve when more sodium hydroxide is added.
| Transition metal ion | Colour |
| Copper(II) | Blue |
| Iron(II) | Green |
| Iron(III) | Brown |
In chemistry, there are many different tests which we can undertake to find out the composition of different substances. These experiments will involve different methods and have a specific result when certain elements are present so that we can measure the presence of these. Here we will look at a variety of different chemical tests, which you must understand for your examination.
Testing for hydrogen
Typically, hydrogen is composed of zinc and sulphuric acid with a bit of copper sulphate solution added to quicken the process. Hydrogen reacts with oxygen when there is a flame or spark, to give water. A lit splint is placed at the top of a test tube of hydrogen and when a ‘squeaky pop’ noise is made, this indicates that hydrogen is reacting with oxygen in the air.
Testing for oxygen
To test for oxygen, we can use our knowledge of this element being flammable. This means that we can take a test tube of oxygen and place a glowing splint into this – the splint will relight in the presence of oxygen. If no oxygen is present, the splint will go out.
Testing for carbon dioxide
A lit splint will go out in a test tube full of carbon dioxide, however, this is also true for other gases, so this test does not ensure we have carbon dioxide specifically. Another test that can be used is to put bubbles of a gas through limewater – if the limewater turns cloudy then you know that carbon dioxide is present.
Testing for ammonia
Ammonia makes damp red litmus paper blue and a white smoke of ammonium chloride is formed when hydrogen chloride gas from concentrated hydrochloric acid is held near it. It has a characteristic sharp smell. Ammonia has a lower density than air and is very soluble in water.
Testing for chlorine
Chlorine has a lower density than air and it is collected downwards into a test tube or gas jar. Chlorine makes damp blue litmus paper turn red which then turns white. It also makes damp starch-iodide paper turn a blue-black.
Testing for sulphate ions
You can see if a solution contains sulphate ions by using barium chloride. Adding barium chloride solution to a sample of water containing sulphate ions will produce barium sulphate. This is insoluble in water and will be a white precipitate.
![Rendered by QuickLaTeX.com \[\text{Barium chloride solution} + \text{Sodium sulphate solution} \rightarrow \text{Sodium chloride solution} + \text{Solid barium sulphate}\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-1f22c5cb39fc2230b0fc27289654cd44_l3.png?lossy=2&strip=1&webp=1)
![Rendered by QuickLaTeX.com \[Ba^{2+}(aq) + SO_4^{2-(aq)} \rightarrow BaSO_4(s)\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-edf72c491fb74a100808fb29159a00b7_l3.png?lossy=2&strip=1&webp=1)
When acid is added to the solution, it destroys other compounds which might form white precipitates when barium chloride solution is added. For example, if acid is not added, a white precipitate will be formed if there was a carbonate present, as barium carbonate is also white and insoluble. The acid reacts and gets rid of the carbonate ions.
Testing for chlorides, bromides and iodides
Halogens of chlorine, bromine or iodine have ions called halide ions. A solution can be tested to see if it contains halide ions (chloride, bromide or iodide ions) by using silver nitrate. If you add silver nitrate solution to a sample of water containing halide ions, the silver halide is precipitated. This is because silver halides are insoluble in water.
Results
| Halide | Precipitate |
| Silver bromide | Cream precipitate |
| Silver chloride | White precipitate |
| Silver iodide | Pale yellow precipitate |
For example
![Rendered by QuickLaTeX.com \[\text{Silver nitrate solution} + \text{Dosium bromide solution} \rightarrow \text{Sodium nitrate solution} + \text{Solid silver bromide}\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-aad534a25734cd334dd1b43b4c0d1d25_l3.png?lossy=2&strip=1&webp=1)
Testing for carbonate ions
This is a test using hydrochloric acid to identify the gas involved. When testing for carbonate ions, limewater is used (CO32-). Acid is added to the test compound and if there are carbonate ions, carbon dioxide gas bubbles off. When this passes through limewater, it turns the limewater from clear to cloudy.
Method:
- Add dilute acid to a solid carbonate and if there are carbonate ions, carbon dioxide gas bubbles will be released.
- Some acid carbonate mixtures can form a salt that does not dissolve and covers the solid carbonate. This stops the reaction. As nitrates are water soluble, if nitric acid was used, this would not happen.
- Add dilute nitric acid and test for gas bubbles that may be formed in the cold. Test the gas with limewater as this will indicate that it is CO2. Below is the equation for this.
![Rendered by QuickLaTeX.com \[CO_3^{2-(s)} + 2H^+ (aq) \rightarrow CO_2 (g) + H_2O (l)\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-0ca69271d8137d7683fa2bd340693d8f_l3.png?lossy=2&strip=1&webp=1)
A physical test for pure water
To test whether the water sample is pure, the boiling and freezing points of the sample can be measured. If they boil at 100 and freeze at 0 at 1 atmosphere, then the sample is pure.
Solutions
Any copper(II) salt in solution will react with sodium hydroxide solution. Below are the equations for this:
![Rendered by QuickLaTeX.com \[CuSO_4 + 2NaOH \rightarrow Cu(OH)_2 + Na_2SO_4\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-940781e49a25f4b0027fec15f1e4a93b_l3.png?lossy=2&strip=1&webp=1)
An orange-brown precipitate forms with iron. This shows that there are iron(III) ions present. The precipitate is iron(III) hydroxide and the equation for this is below:
![Rendered by QuickLaTeX.com \[Fe^{3+} + 3OH^- \rightarrow Fe(OH)_3\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-f59f89eb8a7dc1e9fcd42d011193bdd5_l3.png?lossy=2&strip=1&webp=1)
A green precipitate is formed with iron(III) indicating that iron(III) ions are present. The precipitate is iron(II) hydroxide and the equation for this is below:
![Rendered by QuickLaTeX.com \[Fe^{2+} + 2OH^- \rightarrow Fe(OH)_2\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-f11ed36e7f9e526c5ede1e0b88bce407_l3.png?lossy=2&strip=1&webp=1)
The green precipitate becomes darker and becomes orange-brown at the top of the tube. This occurs because iron(II) hydroxide is oxidised to iron(III) hydroxide by the air.
Cobalt copper chloride paper
This is filter paper that’s been dipped into cobalt chloride solution and dried. When it is dried, the paper turns blue. When water is added to this, it becomes pink. How to make lead iodide (a sample of dry lead sulphate):
- Mix a solution containing lead(II) ions with a solution containing iodide ions:
![Chemical tests \[Pb+^2(aq) + 2l^-(aq) \rightarrow Pbl_2(s)\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-0e914bf179375526245458e582adcf99_l3.png?lossy=2&strip=1&webp=1)
- Lead(II) nitrate is the most common soluble lead(II). Iodide ions would be sodium or potassium iodide solution as all the sodium and potassium salts are soluble.



