In this post
Condensation polymers are produced as a result of a process known as condensation polymerisation. There are different types of condensation polymerisation reactions but for the purposes of this specification we are going to focus on the condensation polymerisation reaction which occurs when a dicarboxylic acid and a diol combine.
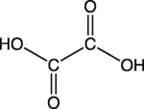
A dicarboxylic acid is a molecule which contains two carboxylic acid functional groups such as ethanedioic acid. Ethanedioic acid has a carboxylic acid functional group at each end of the molecule as shown in the diagram below:
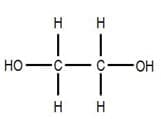
A diol is a molecule which contains two alcohol functional groups such as ethanediol. Ethanediol has an alcohol functional group at each end of the molecule as shown in the diagram below:
An alcohol and a carboxylic acid combine in an esterification reaction to produce an ester. Dicarboxylic and diols will also combine to produce an ester but as each molecule has a functional group at each end, they can form two ester links for each molecule.
When several dicarboxylic acid and diol molecules react together, lots of ester links can be made. Poly means many, so as the resulting molecule contains many ester links, it is known as a polyester.
In a condensation polymerisation reaction, the dicarboxylic acid and diol molecules join together releasing a water molecule for every ester link made.
The hydrogen ion lost from each of the carboxylic acid functional groups and the hydroxide ion lost from each of the alcohol functional groups bond together to form a water molecule. As lots of ester links are being formed, lots of water molecules are given off from the reaction. This production of water is the reason why this process is known as condensation polymerisation.
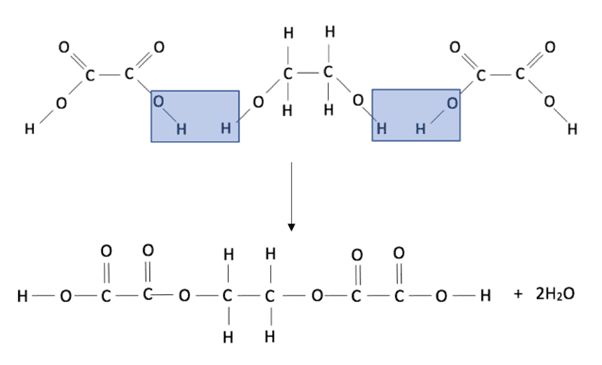
During the condensation polymerisation reaction between a dicarboxylic acid and a diol, a polyester and water are formed as shown in the diagram below using the reaction between ethanedioic acid and ethanediol:
The overall equation for this condensation polymerisation reaction is:
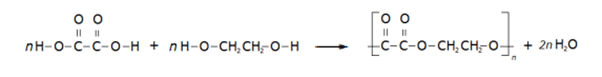
You should be able to write and draw the structural and displayed formula of a polyester, showing the repeat unit from the formulae of the two monomers from which it is formed, as shown in the worked example below.
Example
The dicarboxylic acid ethanedioic acid and the diol ethanediol combine in a condensation polymerisation reaction to produce a polyester.
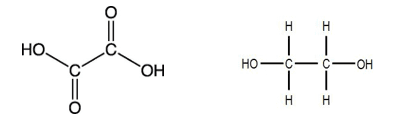
Using the structures of ethanedioic acid and ethanediol shown above, write the structural formula and draw the displayed formula of the polyester produced.
We know that when a dicarboxylic acid and a diol combine, a hydrogen ion is lost from the acid and a hydroxide ion is lost from the diol. The diol and acid combine producing the ester link and the hydrogen and hydroxide ions combine to produce a water molecule from ester links at each end. We only represent one ester link in the repeat unit of the polyester and leave bonds at either end to show where another ester link could be formed, as shown in the diagram below:
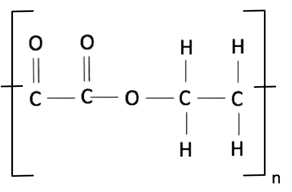
The structural formula for this polyester is therefore (COCOOCH2CH2O) n
Uses of polyesters
Polyesters are very strong and cannot be easily broken down by chemicals. This gives them a wide range of uses including plastic bottles for soft drinks, hard-wearing clothes and fabrics, insulation and cushioning materials, and fabrics for conveyor belts and seat belts.
Some polyesters, known as biopolyesters, are biodegradable
As they are so resistant to chemicals they cannot be broken down easily. Polyesters are also very resistant to being broken down by microorganisms so are not naturally biodegradable. This makes condensation polymers even more difficult to dispose of than addition polymers. To try and overcome this issue, scientists are developing a type of polyester known as biopolyesters.
Biopolyesters are biodegradable. If they are exposed to the air for a prolonged period of time, for example when they are placed in landfill, these polymers will react with the oxygen in the air and decay to form carbon dioxide and water.



