In this post
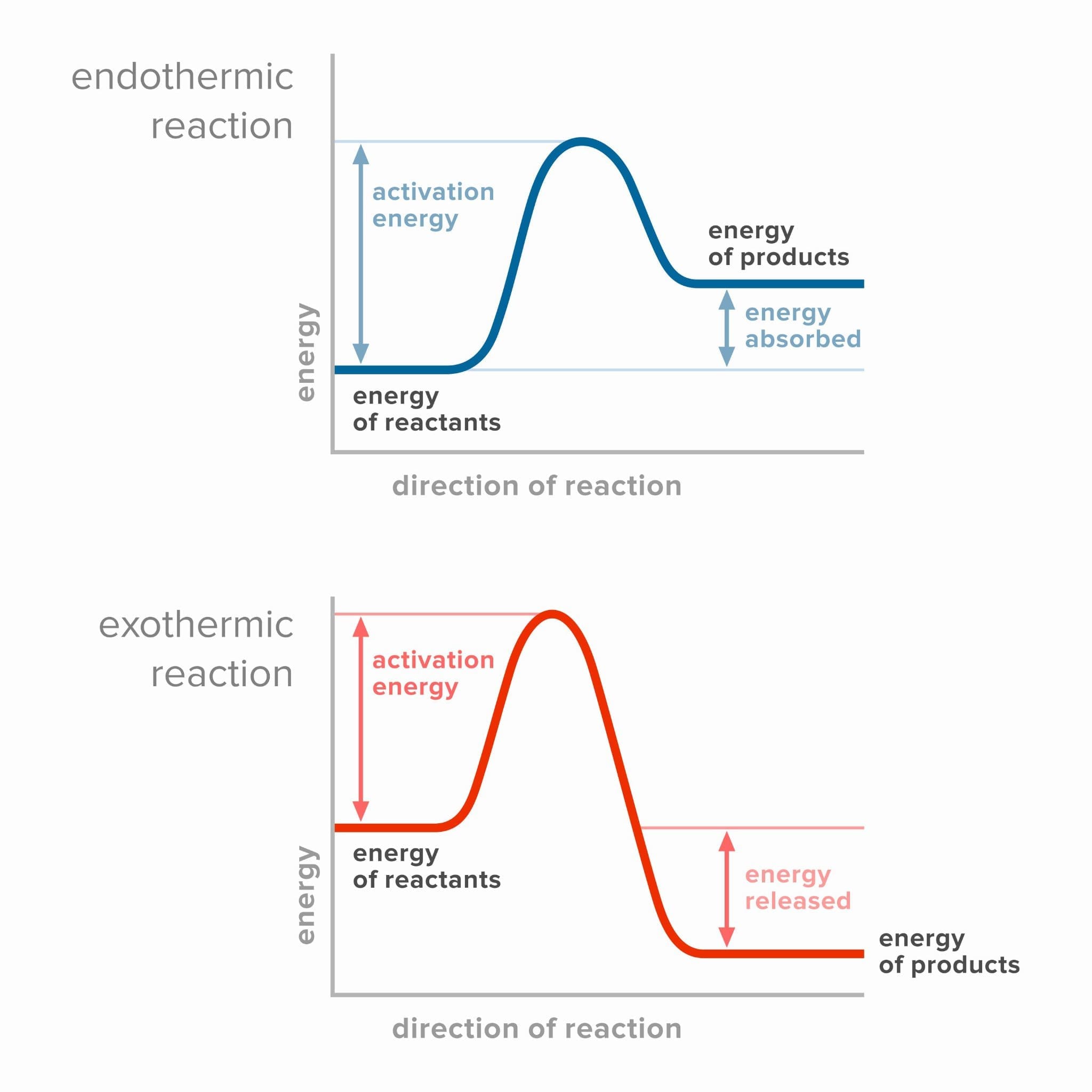
An energy level diagram can be used to represent the energy changes that occur during a chemical reaction. They compare the energy of the reactants to the energy of the products. The difference in energy between the two represents the amount of energy transferred to or absorbed from the surroundings. You must be able to draw and explain energy level diagrams to represent endothermic reactions and exothermic reactions.
Endothermic reactions
In an endothermic reaction, heat energy is absorbed from the surroundings. The reactants start off at a lower energy level. As heat energy is absorbed, in the reaction, the products are at a higher energy level. This means that the reactants will have less energy than the products. The typical energy level diagram for an endothermic reaction is shown below:
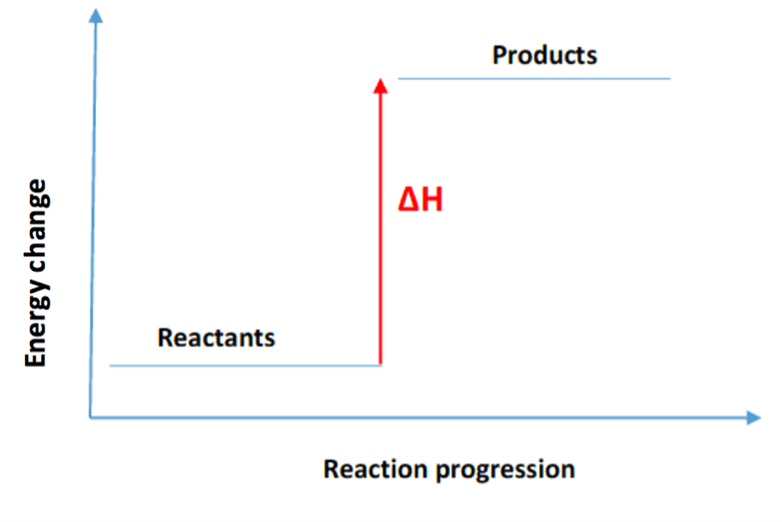
The difference in energy between the reactants and products is the amount of heat energy absorbed from the surroundings. This is represented as ▽H on the diagram.
Exothermic reactions
In an exothermic reaction, heat energy is released into the surroundings. The reactants start off at a higher energy level but as heat energy is lost, the products are at a lower energy level. The reactants have more energy than the products.
The typical energy level diagram for an exothermic reaction is shown below:
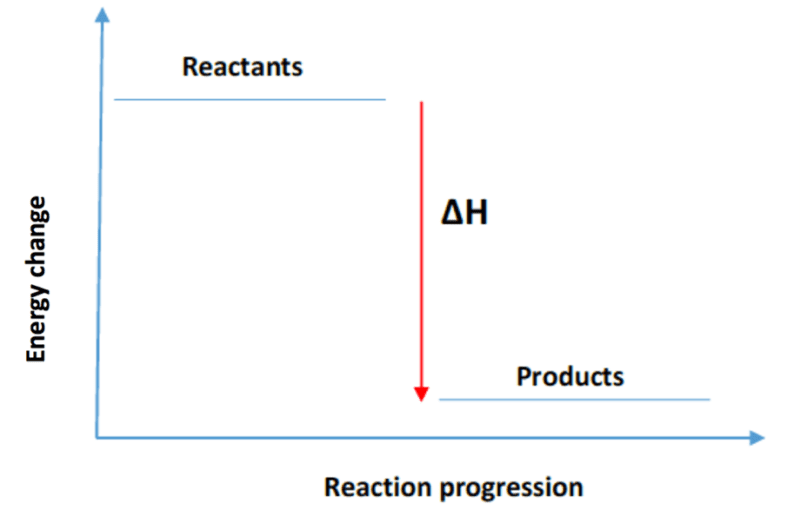
The difference in energy between the reactants and products is the amount of heat energy transferred to the surroundings. This is represented as DH on the diagram.
Bond-breaking and bond-making
In a chemical reaction, the atoms in the reactants are rearranged to form the new products. In order for this rearrangement to occur, the bonds holding the atoms together in the reactants must be broken. Once the atoms have been separated, they can form new bonds to form the products.
Energy must be supplied to overcome the bonds holding the atoms together in the reactants. This energy is absorbed by the reactants from the surroundings. Therefore, bond-breaking is an endothermic process. When the new bonds are formed to make the products, energy is released into the surroundings. Bond-making is therefore an exothermic process.
The amount of energy required to break the bonds can be compared to the amount of energy given out when bonds are made, to evaluate whether the reaction overall will be exothermic or endothermic. If the amount of energy needed to break the bonds is greater than the amount of energy released when new bonds are made, the reaction will be endothermic.
If the amount of energy needed to break the bonds is less than the amount of energy released when new bonds are made, the reaction will be exothermic.