In this post
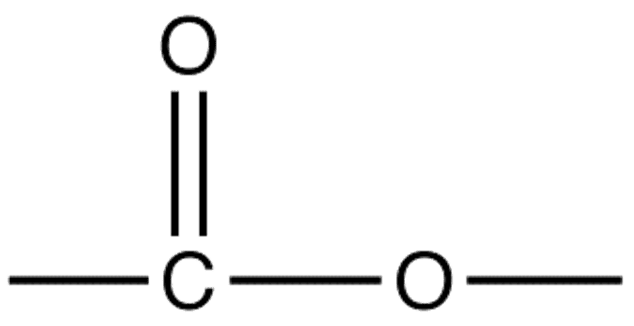
Esters are organic compounds which contain the functional group -COO:
Physical properties
- Esters are colourless, volatile compounds and therefore easily vaporise to turn from colourless liquids to colourless gases
- Esters are also flammable
- Esters are not very soluble in water and are less dense so will form a layer on top of aqueous solutions
- Esters have very distinctive, often sweet-smelling, scents. Some esters have a characteristic smell of pear drops whilst others smell of rum. This property makes them very useful in food flavourings and perfumes
Making esters
Esters are produced during esterification reactions between carboxylic acids, or compounds derived from them, and alcohols. For the purpose of this specification we will just focus on the esterification reaction between carboxylic acids and alcohols.
Alcohols react with carboxylic acids, in the presence of an acid catalyst, in an esterification reaction to produce an ester and water. For example, to produce the ester ethyl ethanoate, a reaction mixture containing ethanol and ethanoic acid and a catalyst of concentrated sulphuric acid, is heated up under reflux.
The ester produced can be observed as a layer formed at the top of the solution, as esters are not soluble in water. The ester layer can be removed from the reaction mixture using the process of distillation.
The water is produced due to the loss of a hydrogen ion from the carboxylic acid molecule and a hydroxide ion from the alcohol molecule. The hydrogen ion and hydroxide ion bond together to form a water molecule.
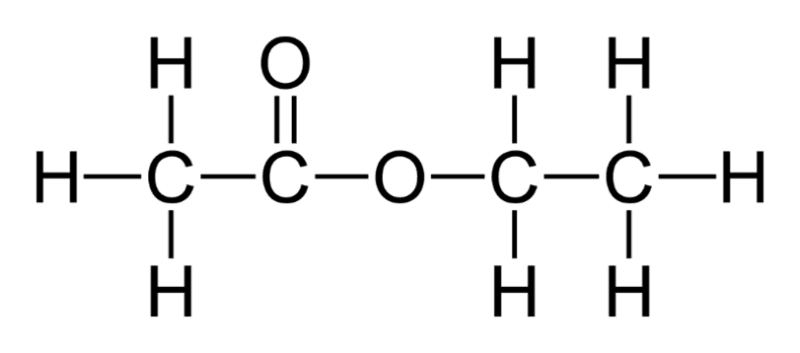
For example, ethanol and ethanoic acid will react in the presence of a sulphuric acid catalyst, to produce the ester ethyl ethanoate and water. The structural and displayed formulae for ethyl ethanoate are shown in the following diagram:
Naming esters
The names of the esters are based on the names of both the alcohol and the carboxylic acid. The first part of the ester’s name comes from the alcohol, with the suffix -anol being changed to -yl. The second part of the ester’s name comes from the carboxylic acid, with the suffix -oic acid replaced by -oate.
The general structure of esters is shown in the diagram below where the carboxylic used is found on the left and the alcohol used is found on the right attached to the oxygen atom of the single C-O bond in the ester link:
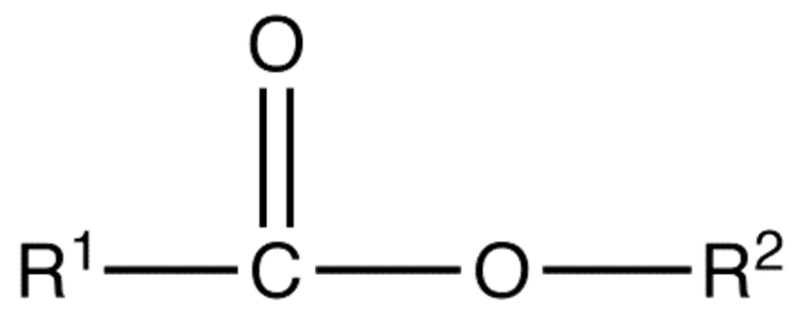
Where R1 is carboxylic acid and R2 is an alcohol.
The structural and displayed formulae for any ester can be written from its name using this general structure. For example, any ester named ethyl propanoate must have been made using ethanol and propanoic acid.
The propanoic acid part of the ester would be drawn on the left on the ester group and the ethanol would be attached to the right of the ester. The structural and displayed formulae for ethyl propanoate would be:
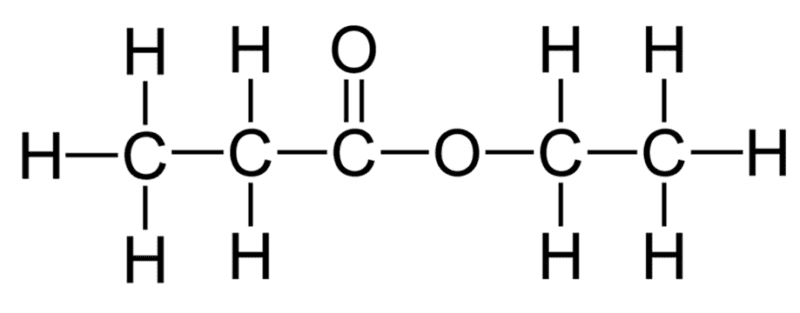
The ester’s name can be determined from its structural and displayed formulae.
For example, to name the ester in the diagram below we must firstly count the number of carbon atoms in each of the carboxylic acid and alcohol chains:
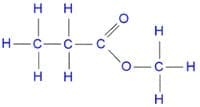
There are three carbon atoms on the left of the ester meaning that the carboxylic acid contained three carbon atoms. The carboxylic acid must therefore be propanoic acid. There is one carbon atom on the right of the ester meaning that the alcohol only contained one carbon atom. The alcohol used must therefore have been methanol. The name of this ester is therefore methyl propanoate.