In this post
The air that we breathe on Earth is a mixture of different gaseous elements and compounds. The gases found in a sample of air can vary depending upon the amount of water vapour in the air and the level of pollution.
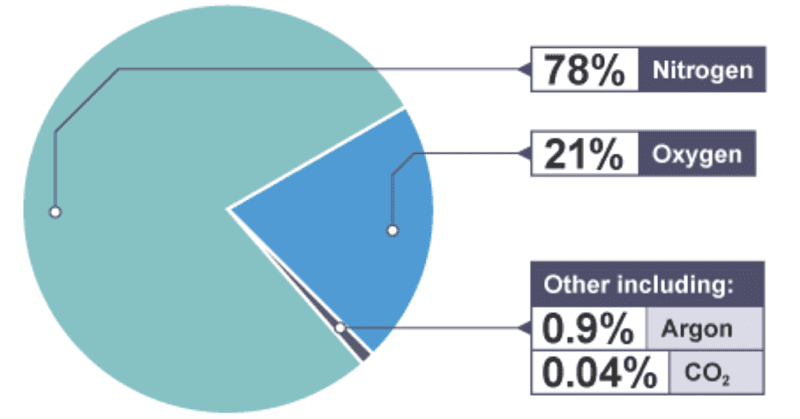
When discussing the approximate percentages by volumes of the most abundant gases we describe the air as being ‘dry air’ where we ignore the amount of moisture present. The four most abundant gases in dry air are nitrogen(N2), oxygen (O2), argon (Ar) and carbon dioxide (CO2). The percentages by volumes of these gases are:
- Nitrogen = 78.1%
- Oxygen = 21.0%
- Argon = 0.9%
- Carbon dioxide = 0.04%
Experiments to show the percentage of oxygen in air
The percentage by volume of oxygen in air can be established using experiments involving the reactions of metals such as iron and non-metals such as phosphorus, with the air.
To do this a known volume of air can be passed over a piece of iron or phosphorus which has been strongly heated. Two gas syringes would be placed at each side with a glass tube containing the sample placed between and connecting the two gas syringes. This set-up is shown in the diagram below using copper metal as the example.
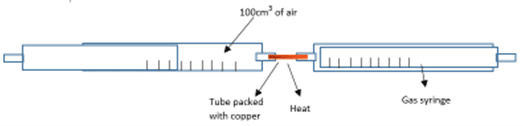
The air is passed backwards and forwards over the hot iron or phosphorus, using gas syringes. The oxygen in the air reacts with the sample to produce iron oxide or phosphorus oxide and the total volume of air reduces as the oxygen from the air is used up. The air would be continually passed over the sample until the volume of air no longer changes, indicating that the oxygen had all been used up.
The volume of oxygen which has reacted, and which was therefore present in the original sample, can be determined by subtracting the final volume of air left from the total volume of air at the start.
This method can be used with any metal. For example, in the diagram showing copper metal being heated, the 100cm3 of air is pushed back and forth over the hot copper using the gas syringes. The oxygen from the air reacts with the copper forming black copper oxide.
As the oxygen is used up, the volume of air in the syringe decreases. The copper is continually heated by moving the Bunsen burner along the metal. Once the volume of air in the syringe has stopped decreasing, all of the oxygen has been used up.
If the volume of air remaining in the syringe was 79cm3, we can calculate the volume of oxygen used was 100 – 79 = 21cm3. This shows that 20% of the air is made up of oxygen gas.
Combustion of elements in oxygen
Combustion is the scientific term used for burning. A combustion reaction is one in which a substance burns in oxygen. If a metal element undergoes a combustion reaction, a metal oxide is produced. The metal oxide always looks different to the metal that was being burned. A flame will be seen. The colour of the flame changes depending upon the identity of the metal.
When magnesium undergoes combustion, it burns with a bright white flame. The flame is so bright that you cannot look directly at it with the naked eye as it could cause damage to your retina.
The magnesium reacts with oxygen to produce a white powdery ash of magnesium oxide. The balanced symbol equation for this reaction is:
![Rendered by QuickLaTeX.com \[2Mg + O_2 \rightarrow 2MgO\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-e366e885cc2b8c1a8a79f418f981f773_l3.png?lossy=2&strip=1&webp=1)
Hydrogen will undergo a combustion reaction with oxygen to produce water, as shown by the balanced symbol equation:
![Rendered by QuickLaTeX.com \[2H_{2(g)} + O_{2(g)} \rightarrow 2H_2O_{(l)}\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-879d96f5d616404778520cc69ca3a105_l3.png?lossy=2&strip=1&webp=1)
The flame produced by this combustion reaction is almost colourless and cannot usually be seen easily. A mixture of hydrogen and oxygen gas can be very explosive. A small version of this reaction is used to test for the presence of hydrogen gas. A lit splint is placed into the gas and, if it is hydrogen, the hydrogen reacts with the heat and oxygen in the air to produce a squeaky pop sound. This is a very small-scale explosion.
Sulphur undergoes combustion reactions with oxygen to produce the colourless and acidic gas sulphur dioxide (SO2), as shown by the equation:
![Rendered by QuickLaTeX.com \[S_{(s)} + O_{2(g)} \rightarrow SO_{2(g)}\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-e3b9953de9ed542c5dbd48e769c28c33_l3.png?lossy=2&strip=1&webp=1)
When sulphur burns in oxygen, a blue flame is produced.
Thermal decomposition
A thermal decomposition reaction is one in which a substance is heated and breaks down (decomposes) into smaller substances. When metal carbonates such as copper(II) carbonate (CuCO3) undergo thermal decomposition reactions, they are broken down to form a metal oxide and carbon dioxide gas. The balanced symbol equation for the thermal decomposition of copper(II) carbonate is:
![Rendered by QuickLaTeX.com \[CuCO_3 \rightarrow CuO + CO_2\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-19160458200d93cc4eaf395f6a001383_l3.png?lossy=2&strip=1&webp=1)
When the copper(II) carbonate is heated, the gas can be collected using a delivery tube and test tube. To confirm the identity of the gas, it can be bubbled through limewater. If carbon dioxide gas is present, the limewater will turn from colourless to a cloudy appearance.
Carbon dioxide contributing to climate change
Greenhouse gases are found in our atmosphere and absorb infrared radiation which is bouncing off from the Earth’s surface. Infrared radiation is produced by the Sun and is sent to Earth providing a heating effect. These greenhouse gases prevent all of the infrared radiation from leaving Earth’s atmosphere and so keep the Earth warm. We do need to have greenhouse gases in our atmosphere to prevent the Earth from getting too cold.
However, if the level of greenhouse gases in the atmosphere is too high, too much infrared radiation will be trapped and the Earth will become too warm. This leads to climate change, polar ice caps melting, flooding and drastic changes in the weather systems.
Carbon dioxide () is a greenhouse gas which remains in the atmosphere for around 100 years. The levels of carbon dioxide in our atmosphere over billions of years have changed naturally due to the evolution of green plants which take in carbon dioxide for photosynthesis and large organisms which expel carbon dioxide as a waste product from respiration.
However, we also increase the levels of carbon dioxide in our atmosphere by burning fossil fuels in power stations to provide electricity and heating for homes and other buildings, running vehicles using petrol and diesel, and deforestation where less carbon dioxide is removed from the atmosphere due to a decrease in the number of trees photosynthesising.
There has been a massive increase in the levels of in our atmosphere since the start of the Industrial Revolution, as we now burn more fossil fuels than ever. The more carbon dioxide there is in the atmosphere, the more heat energy is trapped there. This is thought to be one of the contributing factors to global warming causing climate change.
Oceans can also store carbon dioxide which they absorb from the atmosphere. As the levels of carbon dioxide in the atmosphere increase, so do the levels in the oceans. Carbon dioxide is an acidic gas, so an increase in the level dissolved into the oceans causes the oceans to become too acidic. This increased acidity causes damage to coral and can have a huge effect on the food chain as the organisms relying on the coral for survival start to die out and other organisms that feed on them run out of food.



