In this post
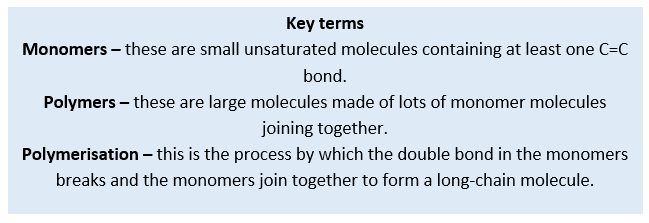
A polymer is a long-chain molecule which is produced by many smaller molecules known as monomers, joining together in a process known as polymerisation. Synthetic polymers are those which are man-made. We can produce different types of polymers by combining different monomers under specific reaction conditions.
Addition polymerisation
An addition polymer is a long-chain molecule which formed by the joining up of many small molecules called monomers. To make addition polymers, the monomers must contain at least one double carbon to carbon bond and are therefore alkenes.
During the process of addition polymerisation, the double bond between the carbon atoms in the monomer alkene molecule breaks open and each carbon is able to form a single bond to a carbon atom in another alkene molecule. No other products are formed, and no atoms are lost.
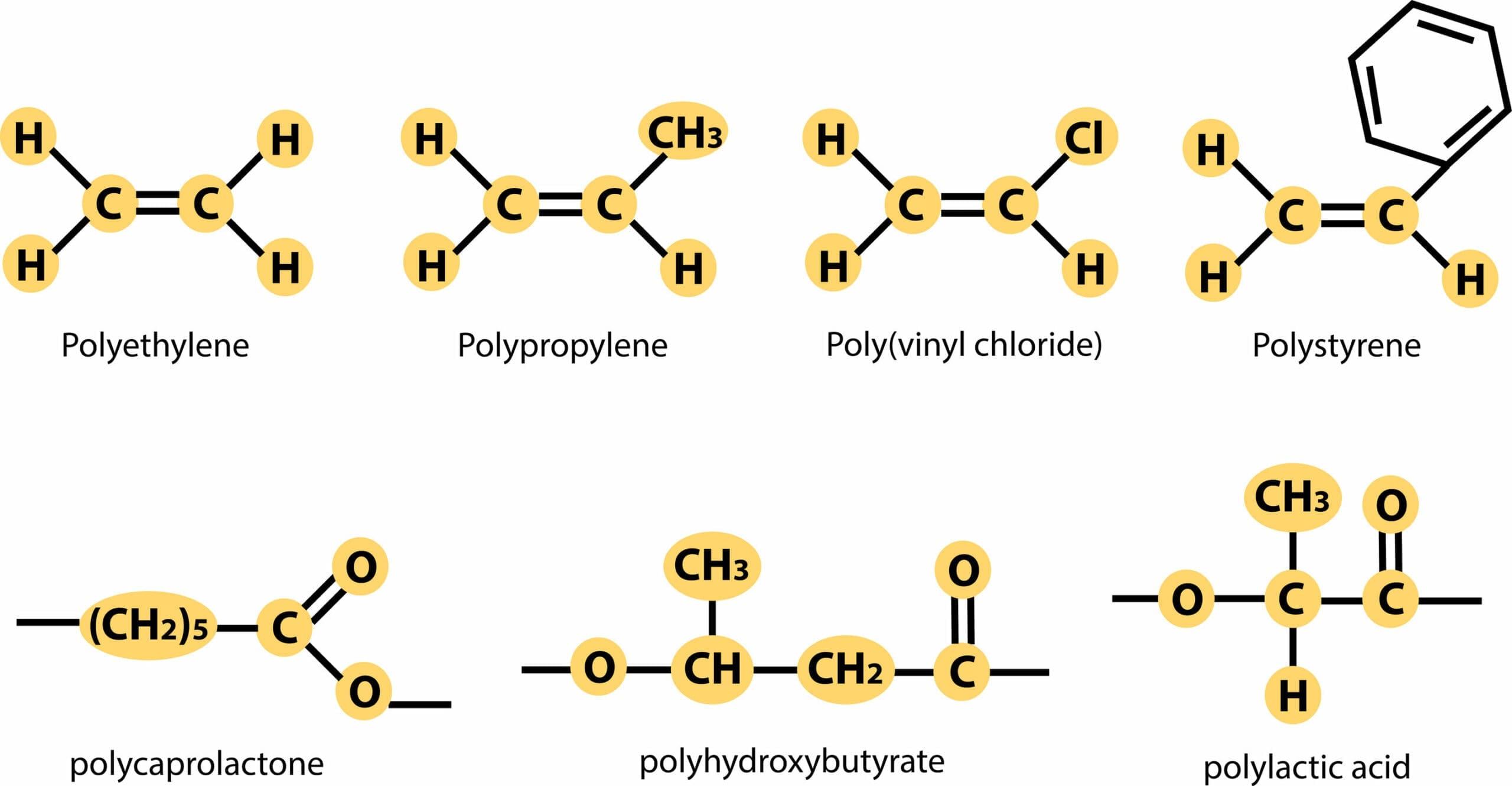
The name of the polymer produced is based on the name of the alkene used as the monomer. The prefix poly- is used, and the name of the monomer given in brackets as the suffix. For example, if the monomer was ethene, the polymer produced would be poly(ethene). The chain length of addition polymers can vary greatly depending upon the number of monomer molecules available. For example, the chain length in poly(ethene) ranges from 4,000 to 40,000 carbon atoms.
As the chain length of the polymer can vary so much, we do not draw the displayed formula. Instead we use a representative diagram which shows the repeat unit of the polymer. The repeat unit is the part of the polymer chain which is repeated over and over again to make the polymer. The identity of the repeat unit is determined by the alkene monomer.
To draw the repeat unit of an addition polymer the double bond in the alkene is changed to a single bond and a single bond is added to each end of the carbon chain. Square brackets are used around the repeat unit and a subscript n placed on the lower right side of the brackets to indicate that there is an unknown number of the repeat units present in the polymer chain. For example, poly(ethene) is made of many ethene molecules joined together. An ethene monomer molecule is shown in the diagram below:
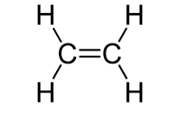
The repeat unit for poly(ethene) is therefore drawn as:
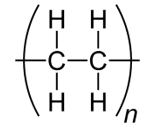
It is possible to identify the repeat unit from a section of a polymer chain and the monomer from the repeat unit, as shown in the worked example below.
Example
A section of polymer A is shown in the diagram below:
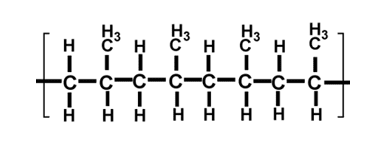
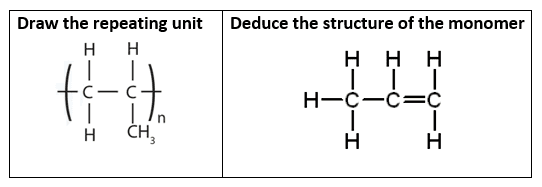
The monomer is propene, so the name of polymer A is poly(propene). Two more common addition polymers are poly(chloroethene) and poly(tetrafluoroethene). The repeat units for each of these are shown below with poly(chloroethene) on the left and poly(tetrafluoroethene) on the right:
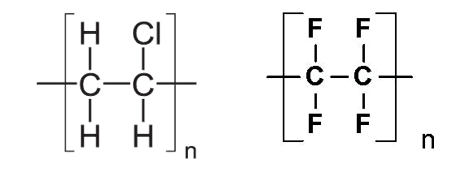
Uses of addition polymers
Addition polymers have a wide range of uses including:
- Window frames and drainpipes
- Insulation and packaging
- Shopping bags and drinks bottles
- Dental fillings
- Non-stick coating of pans and cooking pots
- Clothing
Disposing of polymers
The disposal of addition polymers can pose big problems. Addition polymers are generally unreactive due to the strength of the carbon-hydrogen bonds. This makes them very difficult to break down and, as a result, addition polymers are not biodegradable which means they cannot be broken down by microorganisms in the landfill.
If these polymers are disposed of in landfill, they take up a lot of space and can take hundreds of years to break down. Landfill sites can quickly become too full and overflowing, so alternative forms of disposal need to be considered.
One alternative method which is often used is burning them. As addition polymers contain lots of carbon and hydrogen they make excellent fuels and produce large amounts of heat energy when burned. However, when addition polymers are burned they produce the greenhouse gas carbon dioxide which contributes to enhanced global warming and climate change.
Burning polymers which contain impurities such as sulphur or other elements such as chlorine or fluorine also produces toxic gases such as sulphur dioxide, hydrogen chloride and hydrogen fluoride which can cause serious health issues. One of the best ways to dispose of addition polymers is recycling them. They can be melted down and remoulded to produce other polymer products or we can make use of them in different ways in our homes.