In this post
Carboxylic acids are organic compounds containing the functional group –COOH. The prefix of the names of carboxylic acids are based on the names of alkanes with the same number of carbon atoms in the longest consecutive chain. The suffix of the alkane name -e is replaced by -oic acid. For example, a carboxylic acid containing one carbon atom is named methanoic acid. The names and the structural and displayed formulae for the first four unbranched-chain carboxylic acids are shown in the table below:
| Name | Structural formula | Displayed formula |
| methanoic acid | HCOOH | 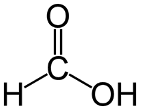 |
| ethanoic acid | CH3COOH | 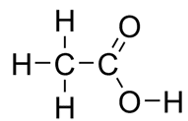 |
| propanoic acid | CH3CH2COOH | 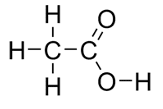 |
| butanoic acid | CH3CH2CH2COOH | 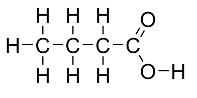 |
Chemical properties
All carboxylic acids contain the same functional group therefore show the same chemical properties. Carboxylic acids dissolve in water to produce solutions which are weakly acidic.
Strong acids are those which fully ionise when added to water. When carboxylic acids are added to water they partially ionise to form an equilibrium, as shown by the equation below using ethanoic acid as our example:
![Rendered by QuickLaTeX.com \[CH_2COOH_{(aq)} \Longleftrightarrow CH_3COO^-_{(aq)} + H^+_{(aq)} \]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-4677b9d85b746f0916bb4e40b50dc4ee_l3.png?lossy=2&strip=1&webp=1)
As the equilibrium for carboxylic acids lies to the left side, only a small amount of H+ ions are released, making the solution weakly acidic. Vinegar is an example of an aqueous solution containing ethanoic acid. This explains why vinegar is weakly acidic.
As carboxylic acids are weak acids, they will demonstrate the same reactions with metals and metal carbonates as the stronger acids we have seen previously. However, the reactions will occur more slowly and will not be as vigorous as they are with the stronger acids.
Reactions with metals
As with all acids, carboxylic acids react with more reactive metals, to produce a metal salt and hydrogen gas. The reactions between carboxylic acids and metals are slower. For example, dilute ethanoic acid reacts with magnesium ribbon to produce the salt magnesium ethanoate and hydrogen gas, as shown in the equation:
![Rendered by QuickLaTeX.com \[2CH_3COOH + Mg \rightarrow Mg(CH_3COO)_2 + H_2\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-23e5885b062bb51dfc89db72c6cda752_l3.png?lossy=2&strip=1&webp=1)
This reaction occurs slowly compared to the same reaction using hydrochloric acid, and it is not always possible to see the reaction occurring. However, if powdered magnesium was used, the reaction would occur much faster which allows observations to be made.
Reaction with metal carbonates
All acids react with metal carbonates to produce a metal salt, carbon dioxide gas and water.
The ionic equation for these reactions is:
![Rendered by QuickLaTeX.com \[2H^+(aq) + CO_3^{2-}(s) \rightarrow H_2O (l) + CO_2 (g)\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-9cc043c23ce5a831bdcd17fc66be2ca4_l3.png?lossy=2&strip=1&webp=1)
Carboxylic acids react with metal carbonates to produce a metal salt, carbon dioxide gas and water but the reaction occurs more slowly than it does using stronger acids such as hydrochloric or sulphuric acid.
The names of the metal salts produced by the reaction with carboxylic acids have a prefix based on the identity of the metal in the metal carbonate and a suffix based on the name of the carboxylic acid used.
For example, if dilute ethanoic acid was added to white sodium carbonate crystals, fizzing will occur immediately as carbon dioxide gas is produced. A colourless solution containing water and the metal salt sodium ethanoate will also be produced. The equation for this reaction is:
![Rendered by QuickLaTeX.com \[2CH_3COOH_{(aq)} + Na_2CO_{3(s)} \rightarrow 2CH_3COONa_{(aq)} + H_2O_{(l)} + CO_{2(g)}\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-941db7849b3014389c9d27548aee6666_l3.png?lossy=2&strip=1&webp=1)