In this post
When a solid (solute) is added to a liquid (solvent) the forces between the solute particles are overcome. This allows the particles of the solute to mix in with the particles of the solvent. This process of mixing is known as dissolving and results in the formation of a solution.
You must understand what is meant by the following key terms:
| Keyword | Definition |
| Solution | This is a combination of a solute and a solvent. |
| Solute | This is the solid that can be dissolved. |
| Solvent | This is the liquid in which the solute will be dissolved. |
| Soluble | Used to describe a substance which can dissolve. |
| Insoluble | Used to describe a substance which cannot dissolve. |
| Solubility | A measure of the amount of solute which can dissolve in a given volume of solvent. |
| Saturated solution | A solution in which no more solute can be dissolved. If any further solute is added, it would be observed as an undissolved solid in the solution. |
The solubility of a solute is measured as the mass in grams which can be dissolved in 100g of solvent at any given temperature. For example, the solubility of aluminium chloride in water is 45.8g per 100g water at 20oC. This means that if 45.8g of aluminium chloride were added to 100g of water at 20oC, a saturated solution would be formed. If any more than 45.8g of aluminium chloride were added, it would remain undissolved in the solution.
The solubility of a solute can be investigated by measuring the maximum mass of solute added in a 100g sample of the solution before the solute starts to be observed as an undissolved solid in the solution.
Working out the solubility of a solute
The solubility of a solute can be determined using experimental results where the volume of the solvent may vary. As long as we know how much of a solute can be dissolved in a certain amount of liquid, we can work out an average for the solubility.
For example, the solubility of sugar at 25oC can be determined by using the results of an experiment where 16g of sugar was dissolved in 250ml of water.
The density of water is 1g/cm3 and 1ml = 1cm3. Therefore, 250ml of water is equal to 250g of water.
- Firstly, we work out how much sugar dissolved in 1g of water by dividing the total amount of sugar dissolved by the total amount of water. (Don’t forget that we have now converted the 250ml of water to 250g.)
![Rendered by QuickLaTeX.com \[\frac{16g}{250g} = 0.064\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-db2f3df0e45a549b96b6ad755d0c4f01_l3.png?lossy=2&strip=1&webp=1)
- Next, we use our answer to part 1 to work out how much sugar would dissolve in 100g of water:
![Rendered by QuickLaTeX.com \[0.064\times 100g=6.4g\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-da10f76d2937dd433ab80fb19843d6a5_l3.png?lossy=2&strip=1&webp=1)
So, this tells us that the solubility of sugar at 25
The solubility that is found in an experiment may not always be accurate and can be affected by three different factors:
- Solvent used – some solvents may be better at dissolving solids than others
- Solute dissolved – some solids may break down easier than others and mix with the solvent easier
- Temperature – the temperature (and therefore energy that is put into the situation) will have a big effect on how much of a solute we can dissolve into a solvent
Solubility curves
At the point when adding more of a solute into the solvent results in the solute not dissolving, the solution is said to be saturated. This means that the solvent has reached the point where it is unable to take any more of the solute and anything else that is added will not dissolve. The mass of solute added to 100cm3 of the solvent before saturation point is reached, can be measured at several different temperatures and the results used to create a solubility curve for the solute.
Solubility curves, such as the one shown below, show the relationship between temperature and solubility.
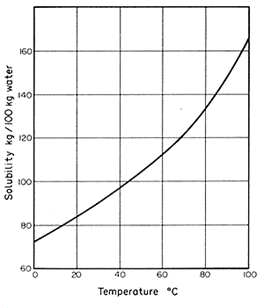
As can be seen on the solubility curve above, as the temperature of the solvent increases, the solubility of the solute also increases.
For example, at a temperature of 20oC the solubility is approximately 85kg/100kg water, whereas at a temperature of 60oC the solubility increases to 112kg/100kg water.
Solubility curves also allow us to predict the solubility of a solution at any given temperature. For example, using the line of the curve we could predict that the solubility of the solution at a temperature of 68oC would be 120kg/100kg water.



