In this post
Chromatography is used to separate colours from mixtures of coloured substances such as inks, dyes and food colourings. A simple chromatography experiment can be carried out using chromatography paper or filter paper. The solvent travels up through the paper and the ink components dissolve into it and are carried up through the paper by the solvent.
The distance travelled by each of the components depends upon their solubility in that solvent. Some components are more soluble so will travel further with the solvent, whereas some are less soluble and will remain on the paper and not travel as far.
As the different components travel at different rates up the paper, this allows them to be separated. The pattern produced from the spreading out of the components is known as a chromatogram and the number of spots seen on the chromatogram indicates the composition of the original mixture.
Figure 1
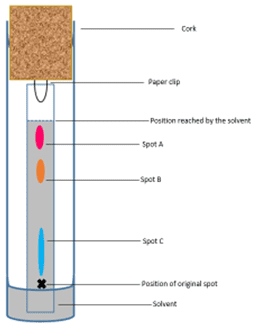
In figure 1, a spot of the original ink mixture was placed at position x and, as the solvent soaked up the paper, was separated into three different spots – A, B and C. This tells us that the original ink was a mixture of three different chemicals. Spot C has barely moved up the paper suggesting it is not very soluble in the solvent. However, spot A has moved as far as the solvent; this suggests it must be very soluble in the solvent. Spot B has moved up the paper a good distance and sits between C and A so its solubility in the solvent must be somewhere in the middle of the other two.
Producing a paper chromatogram
Paper chromatography is used in forensic sciences to match signatures on documents to the pen belonging to a suspect. The method used in paper chromatography is simple but must be followed carefully to achieve accurate results. For example, imagine that a ransom letter has been written and there are three suspects with three different black pens. One of the suspects has written the letter but in order to decide which one, scientists must perform chromatography using the ink from the letter and comparing it to all three pens.
The ink from the pen which was used to write the letter can then be identified as the one which produces a pattern which matches to the pattern produced by the letter ink on the chromatogram.
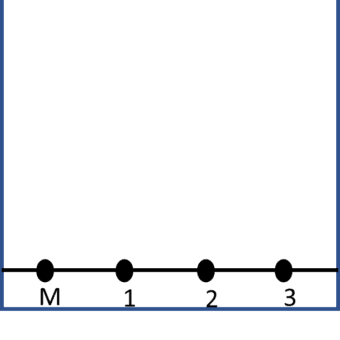
A line is drawn in pencil approximately 2cm up from the bottom of the paper. Ink from the message is then dissolved in the smallest amount of solvent and a spot of this solution is placed onto the pencil line and labelled M. Spots of the inks from the suspects’ pens are also placed on the same line and labelled 1, 2 and 3, as shown below:
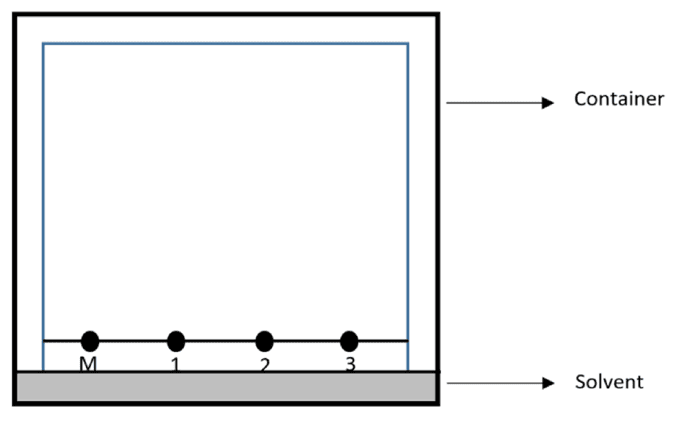
The chromatography paper is then placed into a container containing a shallow depth of solvent. The depth of the solvent must always be lower than the pencil line so that the spots of ink do not wash off the paper into the solvent and are therefore able to travel up the paper with the solvent instead.
A lid must be placed on top of the container to provide an atmosphere which is saturated with solvent vapour. The presence of a saturated atmosphere means that the solvent is less likely to evaporate as it travels up the paper.
The solvent will be absorbed by and travel up through the paper, carrying the different components of the ink mixtures with it. Each component travels at a different rate and is therefore separated into different coloured spots.
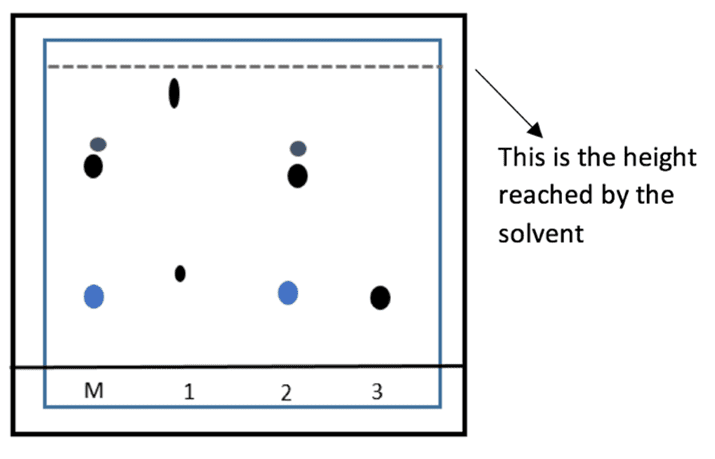
From the chromatogram produced, we can see that the pattern produced by the letter ink can be matched exactly to the pattern produced from suspect number 2’s pen. We can also see that pen 1 contains a combination of two different black dyes due to the presence of two individual spots.
Rf values
While some components travel as far up as the solvent, some stay close to the base line. The components of a mixture can be identified by comparing the distance travelled by the substance to the distance travelled by the solvent. This comparison is known as the retention factor (Rf) value, and is compared to a database of known values to confirm the identity of the component.
The Rf value of a compound always remains the same for any component providing that the chromatography has been carried out in the same way. The equation used to calculate the Rf value is:
![Rendered by QuickLaTeX.com \[ \text{Rf} = \frac{\text{distance moved by the compound}}{\text{distance travelled by the substance}}}\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-3e01b460c3ae61137acca850399faa72_l3.png?lossy=2&strip=1&webp=1)
For example, a component of a mixture travels 8.4cm from the base line and the solvent travels 13.5cm. The Rf value for this component is calculated as:
![Rendered by QuickLaTeX.com \[ \text{Rf} = \frac{8.4}{13.5} = 0.62\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-b80565f6b10980daa6c2cf9ab099f951_l3.png?lossy=2&strip=1&webp=1)
There are no units for the Rf value.



