In this post
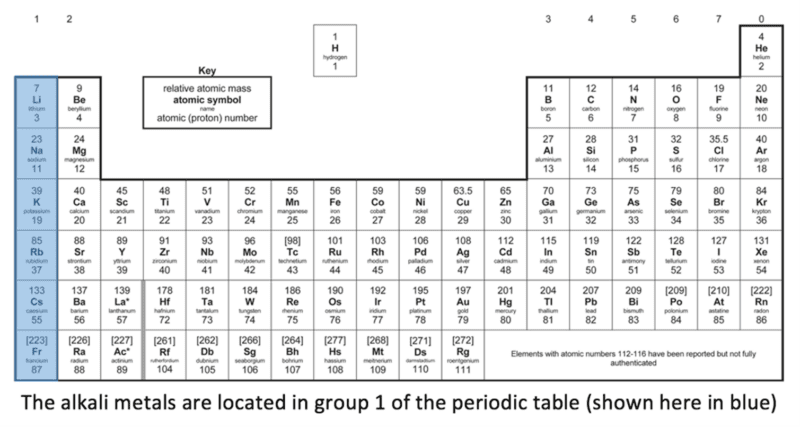
The metal elements in group 1 of the periodic table are known as the alkali metals. They include lithium, sodium, potassium, rubidium and caesium and are shown highlighted in blue on the diagram below:
Physical properties
Elements in the same group of the periodic table show similarities and trends in their physical properties such as melting and boiling points, electrical conductivity and density. The alkali metals all share the same physical properties.
- They are all soft solids at room temperature which can be cut easily with a knife. As we descend the group, the metals become softer and easier to cut
- The alkali metals are all metals with relatively low densities and will float on the surface of water. Their densities increase as we descend the group as the atoms have more mass
- They have very low melting and boiling points which decrease further as you travel down the group
Chemical properties
Alkali metals all show the same chemical properties. They all react with oxygen and water in a similar manner, form colourless solutions of the metal ions, and react with non-metals to produce white or colourless ionic compounds. We will now explore the reactions of alkali metals with oxygen and water in more detail.
Reactions with oxygen
When alkali metals are first cut, their surface is very shiny. This surface quickly becomes dull as the metal reacts with the oxygen in the air. The metal becomes tarnished and a layer of metal oxide is formed. For example, lithium reacts with the oxygen in the air to form lithium oxide:
![Rendered by QuickLaTeX.com \[4Li + O_2 \rightarrow 2Li_2O \]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-b13ff78f944846e438ab4ba48d5dae29_l3.png?lossy=2&strip=1&webp=1)
sodium reacts with the oxygen in the air to form sodium oxide:
![Rendered by QuickLaTeX.com \[4Na + O_2 \rightarrow 2Na_2O \]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-98e2f03983e81ebe7d7741cec26eef16_l3.png?lossy=2&strip=1&webp=1)
and potassium reacts with the oxygen in the air to form potassium oxide:
![Rendered by QuickLaTeX.com \[4K + O_2 \rightarrow 2K_2O \]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-badd2ad820236368ee8cc5f4550fd699_l3.png?lossy=2&strip=1&webp=1)
As you move down through the group, the speed at which the layer of metal oxide is formed increases. This is evidenced by the freshly cut surface of the metal becoming duller much faster. Sodium becomes duller faster than lithium, and potassium becomes duller much faster than sodium.
These observations show that there is a trend in reactivity in group 1. As you descend through group 1, the elements become more reactive with oxygen.
Reactions with water
The alkali metals all have densities which are lower than that of water. This means that when small pieces of them are added to water, they will float. When the alkali metals are added to water they start to react straight away. They move around on the surface of the water and fizzing can be observed. This fizzing shows that a gas is produced. If this gas was tested with a lit splint, a squeaky pop sound would be heard, showing that the gas was hydrogen.
The pieces of metal dissolve into the water to produce a solution. If universal indicator solution was added to the solution formed, a dark blue or purple colour would be observed, showing that the solution formed was strongly alkaline. This is why the group 1 metals are known as the alkali metals.
During these reactions, the alkali metal atoms lose electrons and form metal ions in solution. For example, sodium atoms become sodium ions in solution when a piece of sodium is added to water:
![Rendered by QuickLaTeX.com \[Na_{(s)} \rightarrow Na_{(aq)} + e^- \]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-41f39400a6f038ee2d8ecdf17560d321_l3.png?lossy=2&strip=1&webp=1)
Lithium reacts with water to produce lithium hydroxide and hydrogen gas, as shown by the equation:
![Rendered by QuickLaTeX.com \[2Li(s) + 2H_2O(l) \rightarrow 2LiOH(aq) + H_2(g) \]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-100ebc455d597c81782e9dfcde705e8f_l3.png?lossy=2&strip=1&webp=1)
Sodium reacts with water to produce sodium hydroxide and hydrogen gas, as shown by the equation:
![Rendered by QuickLaTeX.com \[2Na(s) + 3H_2O(l) \rightarrow 2NaOH(aq) + H_2(g) \]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-33e77259876e00195637fbf95d8eaa4c_l3.png?lossy=2&strip=1&webp=1)
Potassium reacts with water to produce potassium hydroxide and hydrogen gas, as shown by the equation:
![Rendered by QuickLaTeX.com \[2K(s) + 2H_2O(l) \rightarrow 2KOH(aq) + H_2(g) \]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-b43000dfe4f5567f5b4d3c2c96752cee_l3.png?lossy=2&strip=1&webp=1)
As can be seen by the equations for the reactions, all of the alkali metals react with, and dissolve into, water to form strongly alkaline solutions of metal hydroxides and hydrogen gas. However, the reactivity of the alkali metals with water changes as you go down through the group.
When lithium is added to the water it moves slowly around on the surface. Fizzing is produced and the piece of metal dissolves slowly. Heat energy is produced but not enough to cause melting or a flame.
When a piece of sodium metal of the same size is added to the water, it moves much faster across the surface. So much heat energy is produced that the sodium melts, forming a ball that moves quickly across the water’s surface. Rapid fizzing also occurs and the hydrogen produced in this reaction burns with an orange flame. The sodium dissolves much faster than lithium.
When a piece of potassium metal of the same size is added to the water, it moves even faster across the surface. In this reaction, so much heat energy is produced that the hydrogen is automatically ignited and the metal also burns, producing a lilac flame. At the end of the reaction, there is often a small explosion. The potassium dissolves much faster than sodium.
As we descend through group 1, the reactions with water become more vigorous, showing that the group 1 metals become more reactive. In the school laboratory we cannot demonstrate rubidium or caesium because their reactions are so vigorous that it would be dangerous to use them. Alkali metals are so reactive with both water and oxygen that they must always be stored under oil to prevent the metal coming into contact with either. Skin contact with alkali metals is dangerous as touching them could lead to a reaction; any sweat on the skin that meets the metal could produce heat and a corrosive metal hydroxide.
Explaining the trend in reactivity
The observations made during the reactions of these elements with oxygen and water, provide evidence for their recognition as a family of elements. They share similarities in the way in which they react with oxygen and water.
The alkali metals are all found in group 1 of the periodic table, which means that they all have one electron in their outer shell.
All elements react to achieve the stability of a full outer shell of electrons and the chemical reactivity of the element is determined by how easily this can be achieved. Alkali metals all need to lose one electron from their outer shell to achieve stability. They do this by reacting with another species which needs to gain electrons.
When the alkali metal atoms lose electrons, they form positively charged ions. The electron configurations for the alkali metal atoms and ions formed are shown in the table below:
| Alkali metal | Atom electron configuration | Ion electron configuration |
| lithium (Li) | 2.1 | [2.]+ |
| sodium (Na) | 2.8.1 | [2.8]+ |
| potassium (K) | 2.8.8.1 | [2.8.8]+ |
As all alkali metals need to lose the same number of electrons, they all behave in a similar way when added to water and oxygen. However, as we descend through group 1, we can see a definitive trend in the reactivity of the alkali metals. The reactions with oxygen and water become more vigorous, showing that the alkali metals become more reactive as we move down the group.
The reactivity of an alkali metal is determined by how easily these outer shell electrons can be lost from the atom. The more easily the outer shell electrons are lost, the more reactive the metal is.
The nucleus of an atom contains protons and neutrons. Protons are positively charged and neutrons have no charge. This means that, overall, the nucleus is positively charged.
There is a force of attraction between the positively charged nucleus and the negatively charged outer electrons. This force of attraction holds the outer electrons tightly towards the nucleus. This force of attraction must be overcome if the outer electrons are to be removed. The closer the outer electrons are to the nucleus, the stronger the force of attraction and the more difficult it is to remove them. The more difficult it is to remove them, the less reactive the element is.
As we move down through group 1, the atoms get bigger and the number of electron shells between the nucleus and the outer shell electrons increases. This means that the outer shell electrons are further away from the nucleus, resulting in the force of attraction between them becoming weaker.
As the electrons are held less tightly by the positive nucleus, they are easier to lose. As we go down group 1, the outer electrons become easier to lose and so the alkali metals become more reactive.
Using trends to predict properties
All elements in group 1 are known as the alkali metals. They all have one electron in their outer shell which they need to lose to achieve stability. This means that they all share similarities in their chemical and physical properties. We also known that there is a trend in the reactivities of the alkali metals where they become more reactive as we descend the group because the outer shell electron becomes easier to lose.
We can use these similarities and trends in reactivities to make predictions about the reactivities of elements lower than potassium in group 1. For example, we can predict that rubidium would react with oxygen to produce rubidium oxide and would react with water to produce hydrogen and rubidium hydroxide solution. We can also predict that both of these reactions would be more vigorous that those of potassium as rubidium is further down the group and its outer shell electron is easier to lose.
Caesium is even further down group 1 so we can predict that it would be even more reactive than rubidium. We can also predict that the melting point and boiling point for caesium would be lower than that of any of the elements above it in group 1, as melting points decrease down the group.