In this post
An ion is a charged particle formed when an atom or molecule loses or gains electrons from the outer shells, causing the number of protons and electrons to become unequal. Protons are positively charged and electrons are negatively charged. If the atom or molecule has lost electrons, the number of protons is greater than the number of electrons. Therefore, the number of positive charges is greater than the number of negative charges and the particle becomes positively charged overall.
If the atom or molecule has gained electrons, the number of electrons is greater than the number of protons. Therefore, the number of negative charges is greater than the number of positive charges and the particle becomes negatively charged overall.
There is always a force of attraction between two oppositely charged ions. This is because of the basic principle of ‘opposites attract’, where any two objects with opposing electrical charges are attracted towards each other. The force of attraction between oppositely charged ions causes them to form an ionic compound. For example, the ionic compound sodium chloride is formed when a positively charged sodium ion is attracted to a negatively charged chloride ion.
Formation of ions
Elements in the periodic table are arranged in groups and periods. The group number of an element tells you the number of electrons it has in its outer shell.
All atoms will react to achieve a full outer shell of electrons. The number of electrons in an element’s outer shell determines the number of electrons it will gain or lose to achieve a full outer shell.
For example, an atom with one electron in its outer shell, will need to either gain seven electrons or lose one to gain the stability of a full outer shell. It takes far less energy and is therefore much easier for the atom to lose one electron than gain seven. If an atom had seven electrons in its outer shell, it would need to either gain one electron or lose seven. In this case it is much easier for the atom to gain one electron that lose the seven from its outer shell. This means that some elements will become ions only by losing electrons and some will become ions only by gaining electrons.
The number of electrons gained or lost determines the charge on the ion. Elements which only lose electrons will only become positive ions and those which can only gain electrons will only become negative ions. The loss of electrons forms positive ions known as cations and the gain of electrons forms negative ions known as anions.
Metal elements in groups 1, 2 and 3 of the periodic table all lose electrons to become metal cations. For example, lithium has one electron in its outer shell which it needs to lose. By losing this electron the lithium atom becomes a lithium ion, which is represented as Li+.
Non-metal elements found on the right-hand side of the periodic table, all have outer electron shells which are almost full. This means that they will always gain electrons to become anions.
For example, fluorine has seven electrons in its outer shell. To achieve a full outer shell, the fluorine atom gains one electron to become a fluoride ion represented as F–.
Representing ions
The overall charge on an ion shows the number of electrons that have been gained or lost to form the ion. Charges on ions are always shown as superscript numbers given after the chemical symbol for the atom or molecule. For example, an oxygen atom could gain one electron to become an oxide ion with a single negative charge which would be represented as , or it could gain two electrons to become an oxide ion represented as. When an atom of magnesium loses two electrons, it becomes a positively charged magnesium ion represented as Mg2+
For your exam, you must know the charges of the ions formed from the metals in groups 1, 2 and 3 and the non-metals in groups 5, 6 and 7:
- Metals in group 1 – all metals in group 1 need to lose one electron, so become ions with a +1 charge. For example, lithium (Li) loses one electron to become Li+, sodium (Na) loses one electron to become Na+ and potassium (K) loses one electron to become K+.
- Metals in group 2 – all metals in group 2 need to lose two electrons, so become ions with a +2 charge. For example, beryllium (Be) loses two electrons to become Be2+, magnesium (Mg) loses two electrons to become Mg2+ and calcium (Ca) loses two electrons to become Ca2+.
- Metals in group 3 – metals in group 3 need to lose three electrons, so become ions with a +3 charge. For example, aluminium (Al) loses three electrons to become Al3+.
Note – when metal elements become ions, their name does not change. For example, a magnesium atom becomes a magnesium ion and a sodium atom becomes a sodium ion.
- Non-metals in group 5 – the non-metals of group 5 need to gain three electrons, so become ions with a -3 charge. For example, a nitrogen (N) atom gains three electrons to become a nitride ion represented as N3- and a phosphorus (P) atom loses three electrons to become a phosphide ion represented as P3+.
- Non-metals in group 6 – the non-metals of group 6 need to gain two electrons, so become ions with a -2 charge. For example, an oxygen (O) atom gains two electrons to become an oxide ion represented as O2- and a sulphur (S) atom gains two electrons to become a sulphide ion represented as S2-.
- Non-metals in group 7 – the non-metals of group 7 need to gain one electron, so become ions with a -1 charge. For example, a chlorine (Cl) atom gains one electron to become a chloride ion represented as Cl–, a bromine atom gains one electron to become a bromide ion represented as Br– and an iodine atom gains one electron to become an iodide ion represented as I–.
Note – when non-metal elements become ions, their name does change. The ending of the element’s name is replaced by the ending -ide as shown in all of the examples above.
Remember
Metal atoms lose electrons to become positively charged ions.
Non-metal atoms gain electrons to become negatively charged ions.
For your exam, you must also know the charges of the following cations:
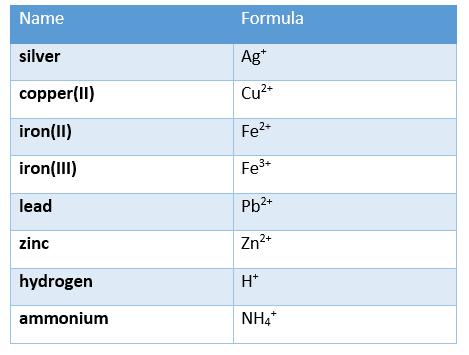
and the charges of the following anions:
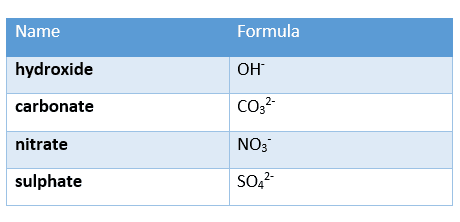
Ionic bonding
When a metal and non-metal react together, the metal loses electrons becoming a cation and the non-metal gains electrons becoming an anion. There is an electrostatic force of attraction between the oppositely charged ions known as an ionic bond.
The formation of these ionic bonds results in the formation of an ionic compound which is always made up of a metal and a non-metal. The name of the ionic compound formed always has two parts. The first part comes from the metal ion and the second part comes from the non-metal ion.
Formulae of ionic compounds
You must be able to write the formulae for ionic compounds formed from all of the ions listed in the previous section. To deduce the formula of an ionic compound, you must consider the individual charges on the metal and non-metal ions. Ionic compounds have no overall charge. Therefore, the overall charges from the metal and non-metal ions must cancel each other out.
Magnesium reacts with oxygen, to form the ionic compound magnesium oxide.
Magnesium has an electron configuration of 2,8,2 so has two electrons in its outer shell which it needs to lose to achieve stability. Oxygen has an electron configuration of 2,6 so it needs to gain two electrons to achieve stability. Each magnesium atom transfers its outer two electrons to the outer shell of an oxygen atom. The magnesium atom becomes a magnesium ion represented as Mg2+. Each oxygen atom accepts the two electrons from the magnesium to become oxide ions represented as O2-. The Mg2+ and O2- ions are attracted to each other and an ionic bond is formed between them.
As the 2+ charge on the magnesium and 2- charge on the oxide ion cancel each other out, we only need one of each of the ions in our overall formula for the compound. Therefore, the formula for magnesium oxide is simply MgO.
If the charges on the ions do not directly cancel each other out, you must consider how many of each ion you need in the formula to make the charges balance as shown by worked examples 1, 2 and 3.
Example 1
Calcium (Ca) reacts with chlorine (Cl) to form the ionic compound calcium chloride. Deduce the ionic formula for calcium chloride.
Calcium is a group 2 metal which loses two electrons to become a calcium ion, represented as Ca2+.
Chlorine is a group 7 non-metal which gains one electron to become a chloride ion, represented as Cl–.
As the charges on the ions do not cancel out, to make the charges balance we need two chloride ions for every one calcium ion. The ionic formulae must represent this ratio.
The number of the metal or non-metal ions needed in the formulae is represented using a subscript number after the symbol. In this example, we would show that we need two chloride ions by using a subscript 2 after the chloride symbol in the formula.
Therefore, the ionic formula for calcium chloride is written as CaCl2.
Example 2
Lithium ions (Li+) bond to carbonate ions (CO32-) to form the ionic compound lithium carbonate. Deduce the ionic formula for lithium carbonate.
Firstly, we need to work out what the ratio of the ions needs to be in the formula to balance out the charges.
Lithium has a single positive charge and carbonate has a double negative charge. Therefore, for every one carbonate ion, two lithium ions are needed to balance out the charge.
The ionic formula for lithium carbonate is therefore Li2CO3.
Example 3
Iron(II) ions (Fe2+) bond to nitrate ions (NO3–) to form the ionic compound iron(II) nitrate. Deduce the ionic formula for iron(II) nitrate.
Firstly, we need to work out what the ratio of the ions needs to be in the formula to balance out the charges. Iron(II) has a double positive charge and nitrate has a single negative charge. Therefore, for every one iron(II) ion, two nitrate ions are needed to balance out the charge. As the formula for the nitrate ion has a subscript number already at the end, it is important to use brackets to avoid confusion and show that there are two nitrate ions present. If the brackets were not used around the nitrate ion, it could appear that there were 32 oxygen atoms in the nitrate group. For example, without the use of brackets the formula would be FeNO32. The correct ionic formula for iron(II) nitrate is Fe(NO3)2.


