In this post
There are many experimental techniques for the separation of mixtures, and we will explore these in this chapter. The techniques used depend upon whether it is the solvent or solute which is desired. Some techniques can only be used if you want to separate and keep the pure solvent, whilst others are more suitable for the separation of the pure solute.
The purity of a substance can be tested by measuring its melting or boiling point. Each pure substance has its own fixed melting point and boiling point. However, a mixture may melt or boil over a range of temperatures. For example, pure water boils at 100℃ but if it contains any dissolved solids, its boiling point will be higher.
Simple distillation
This is a method used to separate a solvent from a solution. The flask containing the solution is clamped into position and placed above a heat source, such as a Bunsen burner. A bung with a thermometer is placed into the neck of the flask with the bulb of the thermometer positioned next to the side arm of the flask where the condenser is attached. The condenser is positioned horizontally diagonally down from the flask with a collecting vessel placed below the opposite end of the condenser to collect the distillate produced, as shown in the diagram on the next page.
An example for the use of this method is for obtaining pure water from seawater. Seawater is a mixture containing salts, water, minerals and various other components and is therefore not safe to drink. However, we can obtain pure water from seawater using simple distillation. Simple distillation works because water has a much lower boiling point than salt. During distillation, the seawater is heated up to the boiling point of water. The water evaporates and forms water vapour, which is then cooled and condenses back to a liquid which can be separated off. The liquid produced is pure water as the salts and other components have been left behind in the flask.
Fractional distillation
Fractional distillation is used to separate a liquid from a mixture of two or more different liquids. The method used for fractional distillation is similar to simple distillation but includes the use of a fractionating column placed between the neck of the flask and the thermometer, as shown in the diagram below:

Fractional distillation can be used to separate ethanol and water. These two liquids are miscible with each other. This means that they can be mixed together to form a single uniform liquid. Their different boiling points allow them to be separated, as water boils at 100℃ and ethanol boils at 78℃.
When the mixture is heated, the ethanol will evaporate first. Some of the water may also evaporate with the ethanol so the temperature of the column must be controlled so that any water vapour formed condenses in the column and drops into the flask. This leads to the alcohol vaporising at the top and out into the container. This means that the ethanol produced first will be pure and should not contain any water.
Once all of the ethanol has been removed, the solution is heated further and the water will evaporate off once its boiling temperature is reached. The water obtained should be pure as all of the ethanol has been removed. This method allows both the ethanol and the water to be collected separately.
Fractional distillation of crude oil
Crude oil is a fossil fuel. It is made up of the fossilised remains of plants and sea creatures which fell to the bottom of the sea millions of years ago. Crude oil is a non-renewable resource which means that once its supplies have been used, it cannot be replaced.
Crude oil is a mixture of hydrocarbon compounds. Hydrocarbon compounds are made up of carbon and hydrogen atoms only. Crude oil itself is not very useful so requires processing to separate the useful hydrocarbon compounds contained. This process is done at oil refineries.

The hydrocarbon molecules are attracted by weak force of attraction which are easily overcome with heat. This means that they can be separated from each other by heating up the crude oil.
In the process of fractional distillation, the crude oil is heated and passed into a fractionating column. The column has a temperature gradient. It is cooler at the top of the column and hotter at the bottom.
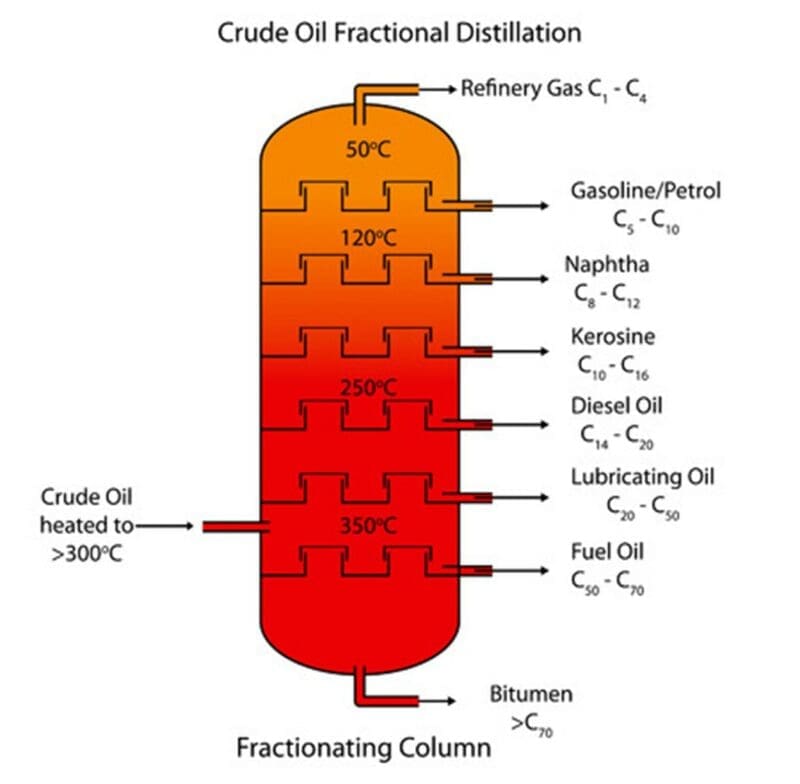
Different hydrocarbons have different boiling points depending upon the number of carbon and hydrogen atoms they have. The longer the molecule, the higher the boiling point will be.
A hydrocarbon with a boiling point of 110℃, would remain as a gas and continue to rise up the column. Once the hydrocarbon has reached the point in the column where the temperature is below its boiling point, it will condense back to a liquid and will be collected on a tray and removed from the column.
As the hydrocarbons all have different boiling points, they will condense, and be removed at, different positions in the column. This allows the separation of different fractions of the crude oil. The temperature at the bottom of the column is not high enough to cause some of the larger hydrocarbons in crude oil to boil. These hydrocarbons therefore remain liquids, do not rise up the column and instead are removed as residue at the bottom of the column.
Fractional distillation of liquid air
To remove dust and impurities, air is filtered and cooled in steps until it reaches -200℃ where it becomes a liquid. During this process, the components of air (carbon dioxide, oxygen, nitrogen) undergo changes and can then be removed from the mixture. Water vapour condenses and absorbent filters are used to eliminate it; carbon dioxide freezes at -79℃ and is also removed; oxygen liquefies at -183℃, and nitrogen liquefies at -196. The liquid forms of oxygen and nitrogen undergo fractional distillation to separate them.
The liquefied air travels to the bottom of a fractionating column where it is much warmer than the top. The liquid nitrogen at the bottom of the fractionating column boils. The gaseous nitrogen rises to the top of the column where it is piped off and stored. The liquid oxygen remains at the bottom of the column.
Filtration
Using the method of filtration, an insoluble solid can be separated from a liquid.
For example, filtration could be used to remove insoluble sand from a mixture of sand and water.
In the method of filtration, a piece of filter paper is held inside a filter funnel made of glass or plastic. The sand remains on the filter paper whilst the water runs straight through.
Crystallisation
Crystallisation is a method used to separate a soluble solid from a solution through evaporation of the solvent. For example, a salt solution can be heated to remove the water by evaporation leaving behind the crystals of pure salt.
Another example of where crystallisation can be used is the production of magnesium sulphate crystals.
Magnesium is added to dilute sulphuric acid forming magnesium sulphate crystals and hydrogen gas.
![Rendered by QuickLaTeX.com \[Mg_{(s)} + H_2SO_{4(aq)} \rightarrow MgSO_{4(aq)} + H_{2(g)} \]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-fee741caf19630e8abcb4538f512b1e5_l3.png?lossy=2&strip=1&webp=1)
Any unreacted magnesium is filtered off from the solution. The resultant solution is heated until crystals form. The crystals formed are surrounded by water molecules which bind to them. These water molecules are known as water of crystallisation and can be easily removed by further heating.
Recrystallisation
This is a process used to purify soluble substances which have been contaminated with small amounts of other soluble substances. The impure solute is dissolved in the smallest amount of hot water and it is cooled again. As most solids are less soluble in cold water than they are in hot water, this cooling results in the crystals reforming. These crystals can then be filtered, washed and dried using filter paper.



