In this post
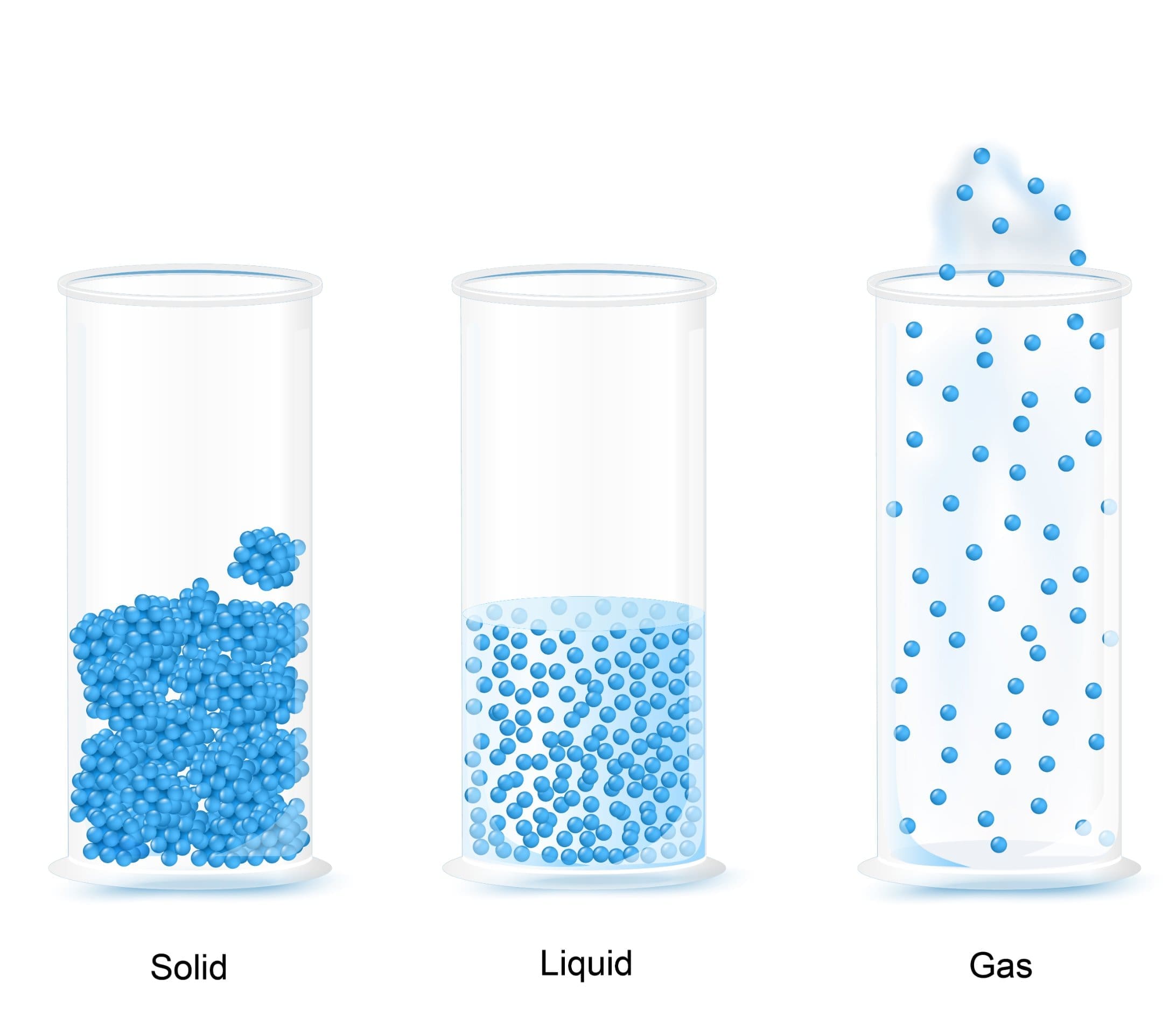
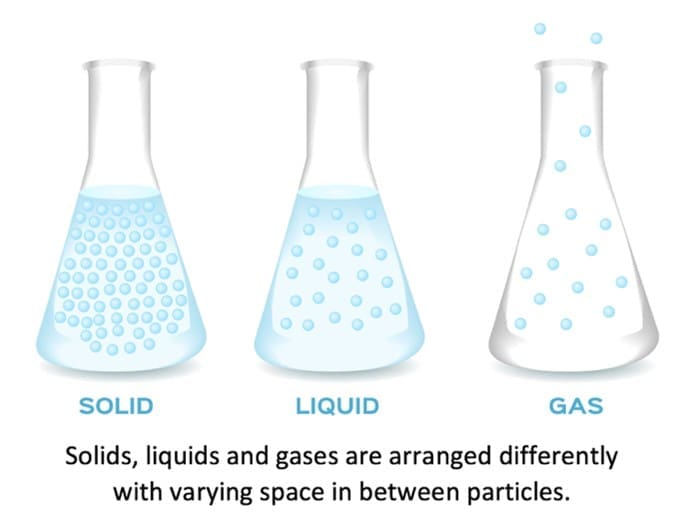
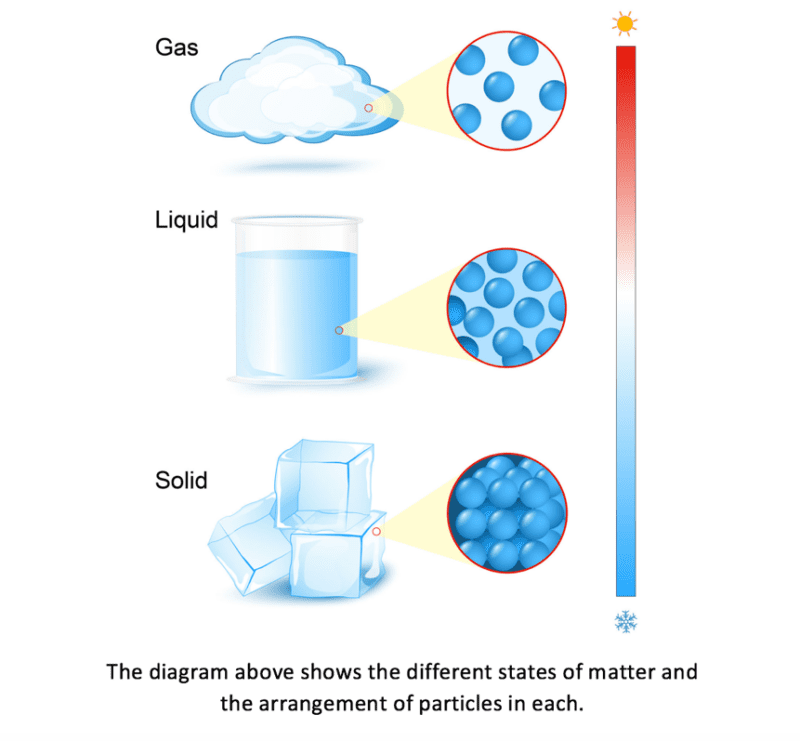
Everything around us is made up of particles that are moving continually. There are three states of matter which you need to understand. They are solids, liquids and gases. The arrangement, movement and energy of the particles contained within these three states of matter differ greatly due to the varying strength of the forces of attraction between the particles in each.
Solids
There are very strong forces of attraction between the particles in a solid. This causes them to be closely packed together with a regular arrangement and fixed lattice shape. The particles in a solid are fixed tightly together. This means that they cannot move or flow past or over each other and explains why solids cannot be compressed or poured from a container. The particles in a solid have little kinetic energy and do not move at all unless energy is supplied. When energy is supplied, the particles are able to move but due to their fixed position, the only movement they have is vibrations in their fixed positions.
Liquids
The forces between particles in a liquid are weaker than those between the solid particles. The particles in a liquid are still relatively close together but there are gaps between them, meaning that the particles are able to move past and over each other more freely and thus allowing the particles to flow to the bottom of a container. This explains why liquids can be compressed and poured. In a liquid, the particles move continually with random motion. When a liquid is heated the particles gain energy and are able to move faster. Therefore, the hotter the liquid the faster the movement. This faster movement causes the forces of attraction between the particles to become weaker. The weaker the forces of attraction between the particles, the further away from each other the particles can move. This can lead to liquids slightly expanding when heated.
Gases
In a gas, the forces of attraction between the particles are very weak so they are able to move very far apart from each other. The large gaps in between the gas particles mean that the particles can move constantly with a random motion. As gases do not have a fixed shape or volume, the particles are able to flow and spread out to fill the container by travelling quickly in different directions. When a gas is heated, the particles gain energy and begin to move faster, leading to the gas expanding. If the gas is expanding in a sealed container with fixed volume, the pressure exerted by the gas increases.
States of matter – interconversions
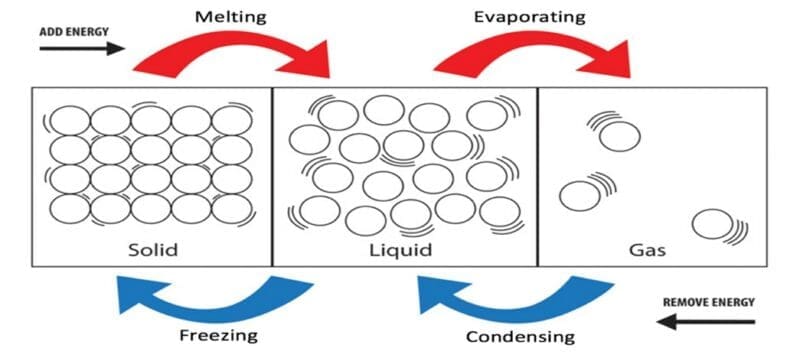
The three states of matter can be converted from one form into another depending upon the amount of thermal energy supplied or removed.
These changes can be observed using water in its three different forms: water as a liquid, ice as a solid and steam as a gas.
Interconversion between solids and liquids
When a solid is heated, the energy is transferred from the thermal energy stores to the kinetic energy stores of the particles. The solid’s particles are then able to vibrate. The more heat energy that is provided to the solid, the more the particles vibrate. This movement weakens the forces of attraction between the particles in the solid. When sufficient heat energy has been provided, these vibrations become so big that the forces of attraction between the particles is weakened so much that the particles are no longer held together. This can cause the solid to expand and change physical state.
For example, if an ice cube (a solid) is heated – a process in which the particles gain more energy – the ice cube will expand and melt to become a liquid. The temperature at which a solid becomes a liquid is known as the melting point of that solid. If the liquid was to be cooled, the particles within it lose kinetic energy and begin moving slowly. The particles get closer together and the forces of attraction between the particles become stronger. The particles are then held in position and form a regular lattice structure. At this point the liquid has frozen forming an ice cube again. The temperature at which a liquid becomes a solid is known as the freezing point of the liquid.
Interconversions between liquids and gases
Changing state between a liquid and a gas can be achieved through condensation and evaporation. When a liquid is heated, the particles gain energy and increase their movement speed. The faster they move, the weaker the forces of attraction between them become and the particles are able to move further away from one another. Eventually, if enough heat energy is provided, the liquid particles move so quickly that they are able to escape from the surface of the liquid, forming steam. This process is known as boiling and the temperature at which a liquid starts to form gas particles is known as its boiling point.
If heat energy continues to be supplied, all of the liquid particles will be able to escape from the container and become a gas. The liquid has been converted into a gas in a process known as evaporating.
If the steam was collected and cooled, the particles in the gas would lose energy and start to slow down. The forces of attraction between the particles becomes stronger and the particles get closer to one another. The gas is converted back into a liquid in a process known as condensing.
Sublimation
Some solids can convert into a gas when heated under specific conditions without becoming a liquid first. For example, solid carbon dioxide (dry ice), becomes a gas at a temperature of -78. These extremely cold conditions cause water vapour to condense in the air forming a white cloud. This shows the conversion of solid carbon dioxide to gaseous carbon dioxide and the process is called sublimation.
These interconversions can only be achieved through the addition or removal of energy from the solid, liquid or gas.
Key terms
Boiling – this is when a liquid is heated so that it becomes hot enough to boil and form gas particles.
Condensing – this involves a gas changing to a liquid.
Evaporating – this involves a liquid changing to a gas.
Freezing point – the temperature that we need for a liquid to become a solid.
Interconversions – the changes between states of matter.
Melting point – the temperature that we need to reach for a solid to become a liquid.
State of matter – the state that a group of particles are in (either a solid, liquid or gas).