In this post
Monitoring the thickness of materials
Industries which use and produce materials such as paper and plastics need to be able to monitor the thickness of the material produced. One way in which they can do this is by using radiation. The source of the radiation is placed on one side of the material whilst the detector is placed on the other side. The thicker the material, the more radiation is absorbed and the less the amount of radiation detected.
Tracing and measuring the flow of liquids and gases
To track the flow of liquids in industrial processes, radioisotopes are used. Radioactive tracers are used to measure how fast sewage is dispersed and can also be used to monitor leaks, as small amounts of radiation can be detected.
Radiocarbon dating
Radiocarbon dating allows the age of a sample to be deduced by testing the level of the isotope known as carbon-14 (C-14) contained within. Carbon-14 is formed from the nuclear transformation of nitrogen gas from our atmosphere. This transformation occurs due to the high energy cosmic rays entering our atmosphere from space. These cosmic rays hit atoms of the gases in the atmosphere and cause the nuclei of the atoms to decay. This nuclei decay causes the sudden release of neutrons which then go on to hit nitrogen atoms causing them to lose a proton and become carbon-14.
The neutron can be represented as:
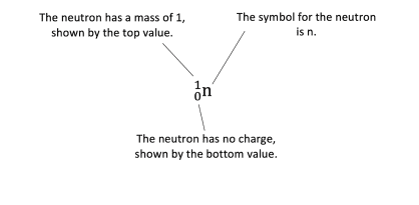
The proton formed can be represented as:
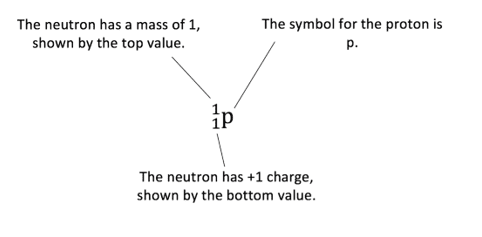
When a neutron collides with a nitrogen atom, the result is a nuclear transformation. The nitrogen atom transforms into an atom of the radioactive isotope of carbon, carbon-14. The nuclear equation for this transformation is shown below:
![Rendered by QuickLaTeX.com \[ \ce{^{14}_{7}N} + \ce{^{1}_{0}n} \rightarrow \ce{^{14}_{6}C} + \ce{^{1}_{1}p} \]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-98519281eb7a8b501e23e5aff15ca0c6_l3.png?lossy=2&strip=1&webp=1)
Isotopes of an element have the same chemical behaviour and the number of electrons is the same. Carbon-14, like carbon-13, will react with the oxygen in our atmosphere to form carbon dioxide. This carbon dioxide is absorbed by plants through the process of photosynthesis. Therefore, a proportion of the carbon that makes up any plant is in the radioactive form of carbon-14. The radioactive isotope carbon-14 that is combined in plant material, enters the food chain. The animals which eat these plant materials also have traces of carbon-14 in their bodies.
When a living organism dies, the radioactive carbon-14 decays over time and the proportion of radioactive carbon in the organic material decreases. Carbon-14 has a half-life of approximately 5,600 years. After every 5,600 years, the amount of carbon-14 remaining in the material will halve.
The amount of carbon-14 present in the organic material can be used to determine its age. To discover how much carbon-14 is available in the sample of dead plant or animal material, the activity of the sample must be measured. This measurement is then compared to the amount of carbon-14 that would have been available when the sample was part of a living organism. This method enables you to calculate and estimate the age of the material. Although this method is useful it also has some issues. By using this method, we have to assume that the amount of cosmic radiation reaching the Earth and being absorbed by the sample, is constant. However, we know that this is not the case as the cosmic radiation has varied greatly over the years. Therefore, to account for this variation, the sample must always be tested and compared to a sample of a known age.
Dating rocks
Inorganic material such as rocks do not absorb carbon-14. This means that carbon-14 isotopes cannot be used to determine the age of rocks. Rocks often contain traces of other radioactive substances such as uranium which also decay to form different isotopes. Sometimes these are isotopes of the same element and sometimes they are isotopes of different elements. The unstable uranium decays to stable lead. The age of the rock can be estimated if the ratio of uranium to lead present, and the half-life of the uranium in the rock, is known. The lower the ratio of uranium to lead, the older the rock is. The half-life of the radioactive isotope can be used to determine the age of the rock. For example, the half-life of uranium-238 is 4.47 billion years. This means that it takes 4.47 billion years for half of the uranium-238 nuclei to decay. If the presence of lead-206 was detected in the rock, at least one half-life must have passed. If the ratio of uranium-238 to lead-206 found in the rock was 1:1, one half-life must have passed since it was formed. This means that the rock is 4.47 billion years old.
| Radioactive parent isotope | Stable daughter element | Half-life (years) |
| Potassium-40 | Argon-40 | 1.25 billion |
| Thorium-232 | Lead-208 | 1.4 billion |
| Uranium-235 | Lead-207 | 704 billion |
| Uranium-238 | Lead-206 | 4.47 billion |
| Carbon-14 | Nitrogen-14 | 5568 |
Medical tracers
Gamma rays are the least ionising form of radiation. They can be used in medicine safely as they mostly just pass through the body without causing too much ionisation in our cells. They also have a very short half-life so any radiation in our body disappears relatively quickly.
Radioactive isotopes can be used in medicine as medical tracers which emit gamma radiation. The isotope is injected into your body and its progress around the body can be tracked by using a gamma ray camera. This allows doctors to check that the movement of the tracer around your body is as it should be.
One example of where these radioactive isotopes are useful is in the diagnosis of thyroid gland problems. The thyroid gland produces thyroxin; a hormone which functions include maintaining heart and muscle function, assisting digestion, brain development and bone maintenance. If the thyroid gland is not able to take in iodine, the production of thyroxin is decreased causing illness. Iodine-123 is used as the medical tracer.
Like iodine-127, the isotope iodine-123 is absorbed by the thyroid gland where it then decays and releases gamma radiation. This can be detected by doctors who are then able to deduce whether the thyroid gland is taking in iodine and functioning as it should.
Treating cancer
Cancer can be treated by eliminating the cancerous cells in the body. This is achieved with the use of chemicals which contain radioactive isotopes. High levels of ionising radiation can kill living cells. However, radiation does not just kill unhealthy cells; it also kills healthy cells. To minimise the damage caused to healthy tissue, this process uses the least ionising form of radiation gamma rays. The gamma rays are directed at the cancer cells specifically and the dosage used is carefully controlled to minimise exposure. This treatment is known as radiotherapy. Unfortunately, some healthy cells will also inevitably be killed off. This is why patients undergoing radiotherapy often feel very sick and unwell.
Sterilisation using radiation
Because ionising radiation kills all cells, it can be very useful in the sterilisation of surgical and medical equipment to kill micro-organisms that could cause infections. This process is known as irradiation or sterilisation. The objects that are to be sterilised are placed close to strongly ionising radiation sources. They must be packed in very airtight bags to ensure that they cannot be re-contaminated by micro-organisms in the air before use. The ionising radiation is able to destroy any micro-organisms present without damaging the item. Irradiation is also used during the preparation of food products to ensure that there are no micro-organisms present in the food to prevent food poisoning.



