In this post
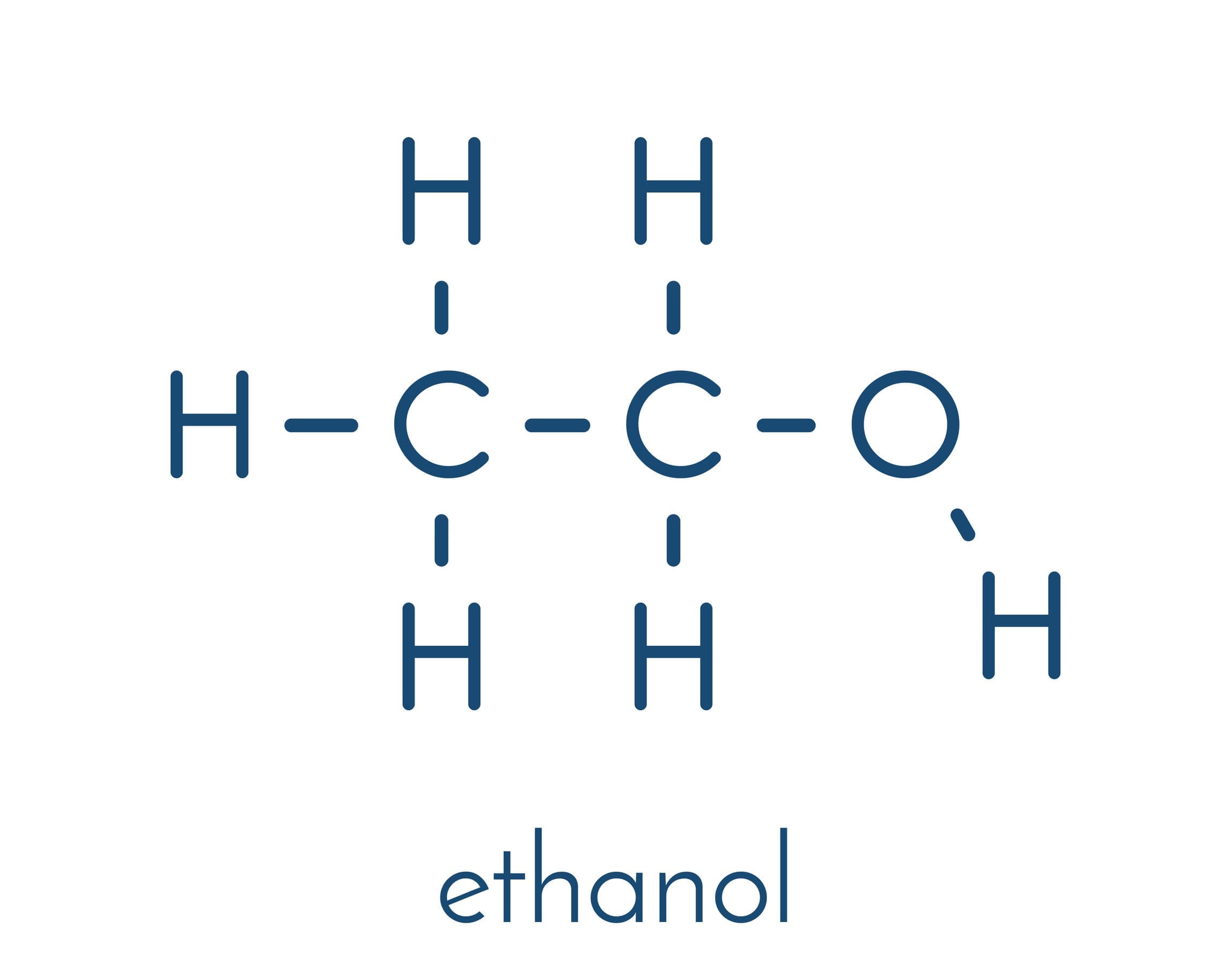
Alcohols are organic compounds containing the -OH functional group. As alcohols contain oxygen as well as carbon and hydrogen, they are not hydrocarbon compounds.
According to the IUPAC rules, alcohols are named using the suffix -ol. The prefix of the alcohol’s name is the same as that of the alkane with a matching number of carbon atoms in the longest consecutive chain. In alcohols the -e on the alkane name is removed and replaced with the suffix -ol. For example, an alcohol containing one carbon atom would be named methanol.
The -OH functional group can be attached to any of the carbon atoms in the chain of the alcohol. This means that alcohols which contain three or more carbon atoms in the longest chain have isomers. The position of the -OH group is indicated using a number between the prefix and suffix of the alcohol’s name.
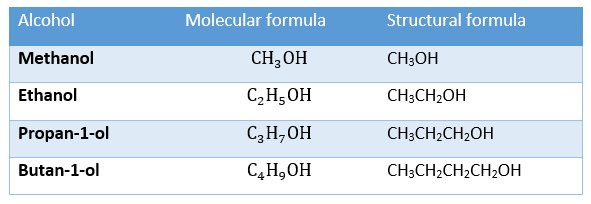
For this specification, we will focus only on the first four alcohols and only on isomers where the -OH group is attached to the first carbon atom in the chain. The general formula for alcohols is CnH2n+1OH Using this general formula, we can deduce the molecular formula for the first four alcohols in the series.
The molecular and structural formulae for the first four alcohols are shown in the table below:
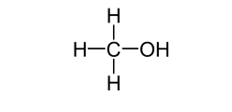
The displayed formula of a compound must include all bonds present. When drawing a displayed formula for an alcohol it is important to remember that you must show the bond present between the oxygen atom and the hydrogen atom in the -OH functional group.
The displayed formula drawn for methanol in the diagram below is incorrect as no bond has been shown between the oxygen and hydrogen:
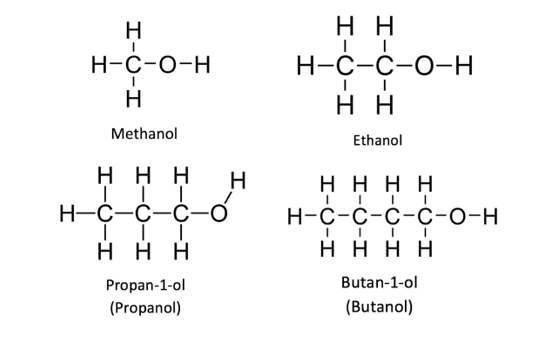
The displayed formulae for the first four alcohols are shown below:
Physical properties of alcohols
The presence of the -OH group in alcohols causes them to behave differently to alkanes and alkenes. Alkanes and alkenes are not soluble in water as the bonds between the carbon and hydrogen atoms are too strong. Alcohols are soluble in water as the -OH group interacts with, and forms intermolecular attractions to, the water molecules. The boiling points of alcohols are higher than those of alkanes and alkenes with the same length of carbon chain. The intermolecular forces between alcohol molecules are much stronger than those occurring between molecules of alkanes or alkenes due to the presence of the -OH group. This means that a higher amount of energy is required to overcome these intermolecular forces and separate the molecules.
Methanol is the simplest alcohol in the series. Methanol is not safe for human consumption as it is poisonous and could cause serious organ damage such as blindness, or even lead to death. Methanol is most commonly used as a fuel or an additive in methylated spirits used for cleaning and removing paint.
Ethanol is used as a solvent in perfumes and aftershaves, as well as in the production of alcoholic beverages such as wines and beers. Ethanol is not poisonous but can cause severe damage to the body if consumed in excessive amounts. For the purposes of this specification we will study the chemical behaviour of the alcohols using ethanol.
Oxidation of ethanol
Alcohols can be oxidised to form carbon dioxide and water in the process of complete combustion. Alcohols are very useful as fuels. They undergo complete combustion in oxygen to produce lots of heat energy. When ethanol (C2H5OH) undergoes complete combustion, it is oxidised to form carbon dioxide and water as shown by the equation:
![Rendered by QuickLaTeX.com \[\text{ethanol} + \text{oxygen} \rightarrow \text{carbon dioxide} + \text{water}\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-65423712619c64e5fa09e3636b916119_l3.png?lossy=2&strip=1&webp=1)
![Rendered by QuickLaTeX.com \[C_2H_5OH + 3)_2 \rightarrow 2CO_2 + 3H_2O\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-c04dc24a3d1ce3ec197d2209907f2942_l3.png?lossy=2&strip=1&webp=1)
Alcohols can also be oxidised to carboxylic acids. Ethanol can be oxidised to ethanoic acid by microbial oxidation or by heating with the oxidising agent potassium dichromate(VI).
Microbial oxidation
Aerobic conditions are those in which oxygen is present. The air around us is made up of approximately 21% oxygen. If ethanol is exposed to aerobic conditions, microbial oxidation occurs where microbes oxidise the ethanol to ethanoic acid (CH3COOH):
![Rendered by QuickLaTeX.com \[C_2H_5OH_{(l)} + O_{2(g)} \rightarrow CH_3COOH_{(aq)} + H_2O_{(l)}\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-25330a9836d689e37cd5298f081368e4_l3.png?lossy=2&strip=1&webp=1)
Vinegar is an aqueous solution containing ethanoic acid. When a bottle of wine is left open and exposed to the air, microbial oxidation occurs causing the wine to turn vinegar-like in taste.
Heating with potassium dichromate(VI)
An oxidising agent is a substance which causes another substance to become oxidised and is itself reduced in the process. Potassium dichromate(VI) is an example of an oxidising agent which is used to oxidise ethanol to ethanoic acid.
In this process the ethanol is placed in a flask and a mixture of dilute sulphuric acid and potassium dichromate(VI) is added.
The sulphuric acid acts as a catalyst for the reaction and is not involved in the chemical changes which occur. The potassium dichromate(VI) is the oxidising agent and causes the ethanol to be oxidised to ethanoic acid. Potassium dichromate(VI) is an orange solution containing chromium ions with a charge of +6. As the ethanol is oxidised to ethanoic acid, the chromium in the potassium dichromate is reduced. The colour of the solution changes from orange to green as the chromium(VI) ions are reduced to chromium (III) ions. The equation for this reaction is below, where the oxidising agent is represented as [O]:
![Rendered by QuickLaTeX.com \[C_2H_5OH_{(l)} + [O] \rightarrow CH_3COOH_{(l)}\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-5dd3475c84d9b180163bdd95e623f8dc_l3.png?lossy=2&strip=1&webp=1)
Ethanol manufacturing
Ethanol is a very useful and efficient fuel and described as ‘clean-burning’. Ethanol is often mixed in with petrol to be used as a fuel in cars, because although it still produces the greenhouse gas carbon dioxide, it increases the efficiency of the combustion, meaning that less incomplete combustion occurs than when petrol alone is used.
Ethanol can be manufactured using ethene from crude oil or glucose from plants such as sugar cane. This means that countries with little or no crude oil supplies, for example Brazil, are still able to produce ethanol by growing sugar cane which can be fermented to form ethanol.
Using ethene and steam
Ethene gas (C2H4(g)) is obtained from the fractional distillation of crude oil. When a mixture of ethene gas and steam (H2O(g)) is passed over a phosphoric acid catalyst at a temperature of 300oC and a pressure of 60-70 atm, ethanol is produced.
This method is relatively cheap but uses the non-renewable resource crude oil as a source of ethene. Supplies of crude oil are running out, so this method cannot be used as a long-term source.
Fermentation of glucose
Ethanol obtained through this method is known as a biofuel as it is obtained through the fermentation of plant material which is a renewable resource. The plants that are used can be replaced by growing new crops.
In the process of fermentation, yeast is added to the plant material under anaerobic conditions and a temperature of about 30oC. Under these conditions, the enzymes in the yeast break down (ferment) the glucose (C6H12O6) in the plant material to ethanol and carbon dioxide, as shown by the equation:
![Rendered by QuickLaTeX.com \[C_6H_{12}O_{6(s)} \rightarrow 2CO_{2(g)} + 2C_2H_5OH_{(l)}\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-37bfa188d92ef78281153751faa07557_l3.png?lossy=2&strip=1&webp=1)
Anaerobic conditions are those in which no oxygen is present. It is important that oxygen is absent because any oxygen present would cause the ethanol produced to be oxidised to ethanoic acid.
The enzymes in yeast will only work at specific temperatures. If the temperature was too low the enzymes may be inactive and would not break down the glucose. If the temperature was too high, the enzymes would become denatured and would not be able to break down the glucose. The temperature should be kept in the region of 300C to keep the enzymes active and avoid them being denatured.