In this post
Crude oil is a mixture of hydrocarbon compounds with differing lengths of carbon chains. Hydrocarbons with different chain lengths have different boiling points. As the different hydrocarbons in crude oil are not chemically bonded to each other, they can be separated by heating the crude oil in a process known as fractional distillation.
In fractional distillation the crude oil is heated, and the vapours passed into a fractionating column which is cooler at the top and hotter at the bottom, as shown in the diagram below:
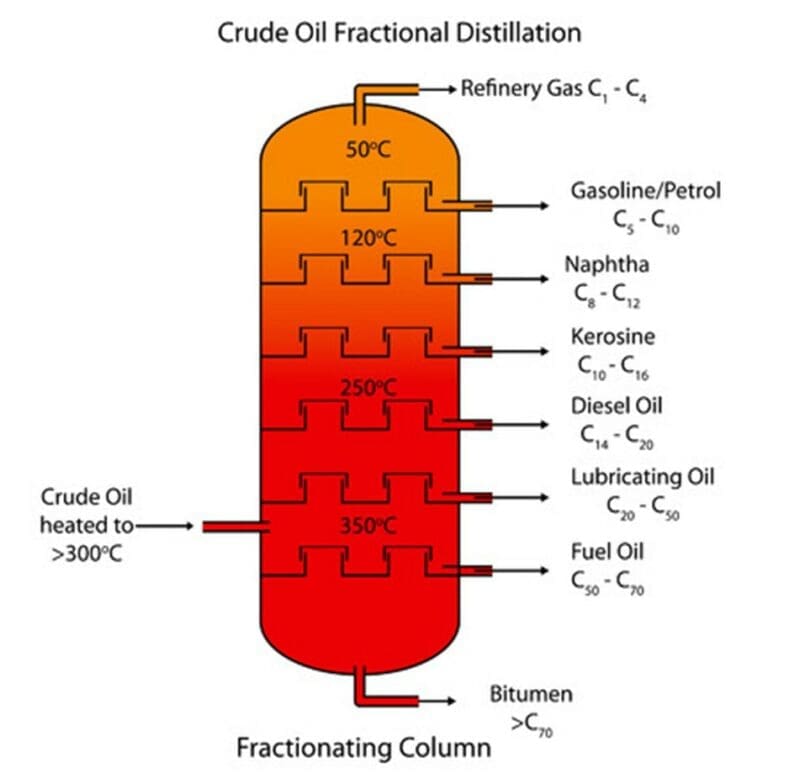
As the hydrocarbons boil, their vapours rise up through the fractionating column where they then condense off at different temperatures. A hydrocarbon with a boiling point of 110 would remain a gas at the bottom of the fractionating column. The temperature towards the top of the column begins to decrease. As the gas rises to the top of the column, it begins to cool down and turn into a liquid. This is known as condensation. The groups of hydrocarbon molecules with similar chain lengths will have the same boiling points and will condense at the same temperature. These groups of hydrocarbons are known as fractions. During the process of boiling, enough energy has to be supplied to overcome the forces of attraction between the molecules and separate them.
Viscosity is a measure of the resistance of a substance to flow. A substance with a low viscosity can flow very easily and a substance with a high viscosity does not flow easily at all. For any substance to be able to flow, the forces of attraction between the molecules have to be overcome. As the number of carbon atoms in a hydrocarbon chain increases, the strength of the intermolecular forces increases. The molecules are held more strongly in place and it takes more energy to separate them.
Therefore, as the number of carbon atoms in the chain increases, the boiling point and viscosity also increases. The main fractions separated from fractional distillation of crude oil are refinery gases, gasoline, kerosene, diesel, fuel oil and bitumen. The boiling points and viscosity decrease with carbon chain length.
Refinery gases have the shortest carbon chains and therefore the lowest boiling point and lowest viscosity. Boiling point and viscosity increases with increasing chain length, from refinery gases to gasoline to kerosene to diesel to fuel oil and finally bitumen. Bitumen has the longest carbon chains and therefore the highest boiling point and highest viscosity. As the carbon chain length of the main fractions increases, the colour becomes darker. The refinery gases are colourless, and bitumen is the darkest coloured fraction. The uses of the main fractions of crude oil are summarised in the table below:
| Fraction | Use |
| Refinery gases | Bottled gas as a fuel |
| Gasoline (Petrol) | Fuel for cars |
| Kerosene | Fuel for aircraft |
| Diesel | Fuel for cars, lorries and buses |
| Fuel oil | Fuel for ships and power stations |
| Bitumen | Road and roof surfacing |
As can be seen from the table above, the majority of fractions separated from crude oil are used as fuels. A fuel is a substance which, when burned, releases heat energy in a process known as combustion.
Combustion
In the process of combustion, a fuel is burned in oxygen to release heat energy. As long as there is sufficient oxygen in the air, complete combustion will take place. If a hydrocarbon compound undergoes complete combustion, carbon dioxide and water are produced. The general word equation for complete combustion is:
![Rendered by QuickLaTeX.com \[\text{fuel} + \text{oxygen} \rightarrow \text{carbon dioxide} + \text{water}}\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-30e984a27dce012e1cc89c953176fb06_l3.png?lossy=2&strip=1&webp=1)
If methane (CH4) reacts with oxygen (O2) in a process of complete combustion, carbon dioxide and water are produced. The balanced symbol equation for this reaction is:
![Rendered by QuickLaTeX.com \[CH_{4(g)} + O_{2(g)} \rightarrow CO_{2(g)} + H_2O_{(l)}\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-f9aef07c801f59c027529521c478172d_l3.png?lossy=2&strip=1&webp=1)
If there is not enough oxygen in the air, incomplete combustion will occur. Incomplete combustion may produce either carbon monoxide or carbon. The general word equation for incomplete combustion producing carbon monoxide is:
![Rendered by QuickLaTeX.com \[\text{fuel} + \text{oxygen} \rightarrow \text{carbon monoxide} + \text{water}}\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-493b62ce804d29a857f785632593ed62_l3.png?lossy=2&strip=1&webp=1)
If methane (CH4) reacts with oxygen (O2) in a process of incomplete combustion, carbon monoxide and water are produced. The balanced symbol equation for this reaction is:
![Rendered by QuickLaTeX.com \[CH_{4(g)} + 1\frac{1}{2}O_{2(g)} \rightarrow CO_{(g)} + 2H_2O_{(l)}\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-92748b5e7a39a85876d3af68232bc1d7_l3.png?lossy=2&strip=1&webp=1)
Carbon monoxide gas produced by incomplete combustion is poisonous. Red blood cells in the bloodstream carry oxygen around the body to the cells where it is used in the process of respiration. If carbon monoxide is inhaled, it is diffused into the bloodstream and binds to the haemoglobin in the red blood cells. This means that the red blood cells are no longer able to bind to the oxygen from the air inhaled and therefore decreases the capacity of blood to carry oxygen.
The general word equation for incomplete combustion producing carbon is:
![Rendered by QuickLaTeX.com \[\text{fuel} + \text{oxygen} \rightarrow \text{carbon} + \text{water}}\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-572d1b8279d3e693c44d620d9dca1157_l3.png?lossy=2&strip=1&webp=1)
If methane (CH4) reacts with oxygen (O2) in a process of incomplete combustion, carbon particles and water are produced. The balanced symbol equation for this reaction is:
![Rendered by QuickLaTeX.com \[CH_{4(g)} + O_{2(g)} \rightarrow C_{(g)} + 2H_2O_{(l)}\]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-9d579ff3550f3baccaec20be493e30fb_l3.png?lossy=2&strip=1&webp=1)
Cracking crude oil
There is an imbalance between the supply and demand for different fractions obtained from the fractional distillation of crude oil. The majority of the hydrocarbon compounds separated through fractional distillation are long-chain alkanes. The vast majority of hydrocarbons are needed as fuels, but most fuels are shorter-chain alkanes. There is a large supply of long-chain alkanes but a large demand for shorter-chain alkanes. There is also a large demand for alkenes as they are used to produce alcohols and polymers such as plastics.
To solve this imbalance between supply and demand, the long-chain alkanes can be converted into shorter-chain alkanes and alkenes through a process known as catalytic cracking.
In catalytic cracking the large hydrocarbon molecules are split down into smaller ones using a high temperature and a catalyst. The long-chain hydrocarbon molecules are passed over a silica or alumina catalyst at a high temperature in the range of 600-700oC. This results in the thermal decomposition of the long-chain molecule to produce one or more shorter alkanes and one or more alkenes.
For example, if the long-chain alkane tridecane (C13H28) was passed over a silica catalyst at a temperature of 650oC, thermal decomposition would occur. The products of this thermal decomposition reaction can vary greatly. Possible products of this thermal decomposition include ethene (C2H4(g)), propene (C3H6(g)) and octane (C8H18(l)) as shown by the equation below:
![Rendered by QuickLaTeX.com \[C_{13}H_{28(l)} \rightarrow C_2H_{4(g)} + C_3H_{6(g)} + C_8H_{18(l)} \]](https://b3801007.smushcdn.com/3801007/wp-content/ql-cache/quicklatex.com-293a6e00960f6ab3ff5b79b2cf4a35b4_l3.png?lossy=2&strip=1&webp=1)
For your exams you don’t have to learn these equations, but you do need to be able to describe how long-chain alkanes are converted to alkenes and shorter-chain alkanes.



