In this post
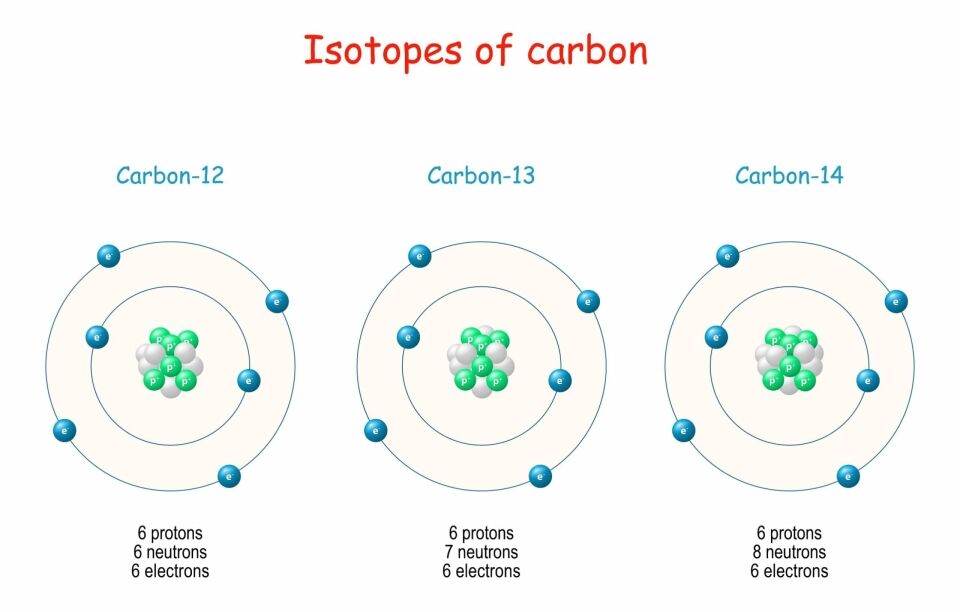
Isotopes are atoms of same element, which have the same number of protons but a different number of neutrons. This means that isotopes have the same atomic number, but different mass numbers. For example, carbon can exist in three different forms with three different mass numbers – carbon-12, carbon-13 and carbon-14. Each of the three isotopes has an atomic number of 6 so each has 6 protons; carbon-12 has 6 neutrons, carbon-13 has 7 neutrons and carbon-14 has 8 neutrons. The different number of neutrons does not affect their chemical reactions, so isotopes will have similar chemical properties. As their masses differ, isotopes may also differ in their physical properties.
Relative atomic mass (Ar)
Relative atomic mass (Ar) is a weighted average of the masses of all of the isotopes of an element. The relative atomic mass of an element is calculated using the mass and relative abundance of each isotope present in a sample of the element. Relative abundance is how much there is of each isotope in comparison to the full amount of the element in the world. The equation used to calculate the Ar of an element is:
total abundance of all isotopes present
Example
Chlorine has two isotopes – chlorine-35 and chlorine-37. A sample of chlorine gas was made up 75% chlorine-35 and 25% chlorine-37.
Use this information to calculate the Ar of chlorine.
Note: there are no units for Ar values